Biography
Interests
Orji, S. L.1*, Ewansiha, J. U.2 & Orji, I. G.3
1Bio-resources Development Centre, National Biotechnology Development Agency, Lugbe Abuja, Nigeria
2Department of Microbiology, Federal University of Technology, Minna, Niger State, Nigeria
3Department of Food Science and Technology, University of Ibadan, Oyo State Nigeria
*Correspondence to: Dr. Orji, S. L., Bio-resources Development Centre, National Biotechnology Development Agency, Lugbe Abuja, Nigeria.
Copyright © 2019 Dr. Orji, S. L., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The quest for nontoxic feed for animal as an alternative to soybean meal cannot be over emphasized considering its high cost and level of demand. The detoxification of Jatropha curcas kernel meal for animal feed using different microorganisms in order to determine the organism with the best fermentative ability, better yield of nutritional value and potential for elimination of anti-nutritional factors was done using standard laboratory methods. The effects of fermentation duration for 5 and 10 days on anti-nutritional components in Jatropha curcas kernel meal were compared to the control meal (unfermented meal). The anti-nutritional composition of Jatropha kernel meals fermented for 5 and 10 days shows no significant difference (p > 0.05), except for tannin, phytate and phorbol ester which were significantly reduced (p < 0.05) from 1.18±0.08, 2.28±0.18mg/g and 0.33±0.14mg/g on the fifth day to 0.98±0.04, 1.72±0.13mg/g and 0.22±0.01mg/g on the tenth day respectively. Microorganism used for the fermentation of Jatropha kernel cake, demonstrate B. subtilis with the lowest concentrations of tannin (0.67±0.13mg/g), saponin (4.50±0.31mg/g), lectin (21.22±2.11mg/g) and phorbol ester (0.12±0.01mg/g) (p < 0.05). However, Aspergillus brasiliensis, and Zymomonas mobilis generated the lowest concentrations of trypsin (25.71±5.80mg/g), phytate and (1.18±0.16mg/g) respectively in J. curcas cake. The solid state and submerged treated Jatropha kernel cake shows no significant difference (p > 0.05) in concentration of anti-nutritional factors, while a significant decrease (p < 0.05) in concentration of anti nutrients was observed in J. curcas kernel cakefermented by both solid state and submerged process when compared with the control. The research findings reveals that microorganisms used in this study could be used for detoxification and improvement of nutritional values of Jatropha curcas kernel meal for animal feed with time optimization.
Introduction
Jatropha curcas is an oil plant belonging to the Euphorbiaceae family [1]. It originated from Central America
but can now be found throughout the tropics including Africa and Asia [2], it is a multi-purpose and large
drought resistant plant with several attributes, considerable potentials, evoked interest all over tropics as a
potential biofuel crop and it Feed represents the major cost of poultry production and the cost of feed ingredients
could be as high as 80% of the total cost of the finished feed [3]. Inadequate production of feeds has
been found to be one of the major factors limiting the development and expansion of poultry business [4].
The world population is increasing and there is an urgent need to increase animal production in order to meet the increasing demand of animal protein. However the consumption of conventional feedstuffs like soybean, maize sorghum etc by human beings is undermining their availability to animals [5]. Hence, the world is becoming increasingly aware of the looming food scarcity, the possibility of raising animal on the unconventional but easily sourced and available feedstuffs in the tropics and subtropics deserves more attention [6].
The use of Jatropha curcas meal in animal nutrition is however faced with several problems of anti-nutritional factors such as; lectin, saponin, tannin, phytate, trypsin inhibitors and phorbol esters [7]. Due to these phytotoxins, the seeds, cakes or its oil cannot be used for human or animal consumption. Also, the disposal of the Jatropha kernel meal (JKM) could be a major environmental problem in the future, due to the generation of huge quantity of JKM after biodiesel extraction. Physical methods and chemical treatment have been used to detoxify the toxic compounds in Jatropha curcas kernel meal [8]. However, the biological method (microbial fermentation) of detoxification is still at its infancy and would be more advantageous than the others because it is environmental friendly, safe, cost effective and conserve energy. Therefore these research is aimed at comparing the submerged and solid state techniques in the fermentation and detoxification of JKM by microorganisms.
Materials and Methods
Media and isolates used for this study include; potato dextrose agar (PDA), nutrient agar and Aspergillus
brasiliensis, Saccharomyces cerevisiae and Candida tropicalis, Penicillium album and Rhizopus microsporum, Bacillus subtilis, B alvevi, B licheniformis, B megaterium, and Zymomonas mobilis respectively;
all the test organisms were isolated from food products; while the chemicals used were standard analytical
grade, purchased from reputable company.
Matured seeds of Jatropha curcas were purchased from River Basin Farms, Minna, Niger State Nigeria.
The seed was identified at the Department of Crop Production, Federal University of Technology, Minna.
These were sorted to get rid of stones and other debris. The cleaned seeds was weighed and later cracked
individually to remove the kernel which was equally weighed. The kernel was milled using miller and defatted
using solvents as described by Belewu, (2008) [9].
A 10 fold and 9 series (10-1 to 10-9) serial dilution of the food product (fermented locust bean and palm wine)
was carried out using sterile test tubes. One mililiter of the 6th (10-6) and 10-2 (fresh palm wine) dilution was
introduced into petri dish and 19ml of Nutrient agar (NA) was added (pour plate method) for the isolation
of bacteria and Potato Dextrose agar (PDA) for the isolation of fungi. The inoculated plates were incubated
at 37ºC for 24 hours for bacteria, while the Potato Dextrose agar (PDA) was incubated at room temperature
(28 ± 2ºC) for 3-5 days. Selected colonies were sub-cultured repeatedly on freshly prepared media used for
primary isolation to obtain pure isolates. The isolates were preserved in NA and PDA slants and kept in the
refrigerator at 35ºF (1.6ºC) for further characterization and identification.
An overnight culture of the test organisms were Gram stained, Gram positive and Gram negative organisms
were recorded. A control smear of known Gram positive organism (Staphylococcus aureus) and a known gram
negative organism (Escherichia coli) was stained simultaneously to confirm the accuracy of the procedure
[10].
The biochemical tests such as Catalase test, Citrate utilization test, Coagulase test, Starch hydrolysis, Methyl
red test, Voges proskauer, Growth at 55ºC, Motility test and Acid & gas production from fermentable sugars
of microbial isolates were carried out according to the methods described by Cheesbrough (2002) [10].
The fungal isolates were identified by direct microscopic observation. This was achieved by the preparation
of smear with grown fungal isolate on clean grease-free slide and a drop of lactophenol blue was added and
the smear was covered with cover slip. The film was viewed under the microscope using the X10 objective. The characteristics observed were compared to that of known taxa. Sugar fermentation was also carried out
on the fungi isolates similar to determine their fermentative characteristics.
Solid State Fermentation (SSF) was carried out using one hundred grammes (100g) of deoiled J. curcas
kernel meal moistened with ten millilitre (10ml) of distilled water in 500ml Erlenmeyer flasks having initial
moisture content of 70% and autoclaved. Samples were inoculated with each of the organism (10
One hundred grammes (100g) of deoiled J. curcas kernel meal and one hundred millilitre distilled (100ml)
of water was mixed to form a broth media in Erlenmeyer flasks of five hundred millilitre capacity and
autoclaved. The sterile contents were inoculated aseptically with each of the organism (106cfu/mL mcfarland
for bacteria isolates) singly (Aspergillus niger, Saccharomyces cerevisiae and Candida tropicalis, Penicillium album
and Rhizopus microsporum, Bacillus subtilis B. alvevi, B. licheniformis, B. megaterium, and Zymomonas mobilis).
The inoculated samples were homogenized and incubated at ambient temperature (fungi) at 37ºC (bacteria)
for ten days on a rotary shaker operated at hundred and fifty revolution per min (150rpm). The flasks was
sampled at days 0, 5 and 10days to determine the toxin and anti nutritional factors. The reduction (%) of
toxic and anti nutritional factors was calculated from different samples in day 0, 5 and 10days. All experiment
was conducted in triplicate. The samples were oven dried at 70ºC for for four days and milled to fine powder.
The sample with the least toxic component from the two methods was selected for further studies.
Phorbol esters in Jatropha curcas seed kernel meals were extracted by the method described by Makkar et al.
(2007). Five grams (5g) of Jatropha Curcas seed kernel meals sample was weighed and the phobol ester was
extracted four times with 150ml of methanol at room temperature maintaining a flow rate of 1.6ml/min in
a gradient elution. The result was determined using a UV spectrophometer at 280nm and the phorbol ester
concentration was expressed as mg/g of meal extracted.
The determination of saponin was carried out according to the method described by Oloyed, (2005) [11].
Half gramme (0.5g) of the sample was added to twenty milliliters (20ml) of 1N HCl and was boiled for
4 hours. After cooling it was filtered and 50ml of petroleum ether was added to the filtrate for ether layer
and evaporated to dryness. Five milliliters (5ml) of acetone ethanol was added to the residue and 0.4mls of
each was taken into three different test tubes. Six milliliters (6ml) of ferrous sulphate reagent was added into
them followed by two milliliters (2ml) of concentrated H2SO4. It was thoroughly mixed after 10 minutes
and the absorbance was taken at 490nm. Standard saponin was used to establish the calibration curve.
The determination of Tannins in Jatropha curcas meal was carried out according to the method described by
Krishnaiah et al., (2007) [12]. Half gram (0.5g) of Jatropha curcas sample was weighed into a beaker and fifty
milliliters (50ml) of distilled water was added and allowed to stand for two hour. The sample was filtered
into a fifty milliliter (50ml) volumetric flask and made up to mark. Five milliliters (5ml) of the extract was
then pipetted into a test tube and mixed with two milliliters (2ml) of 0.1M FeCl3 in 0.1M HCl and 0.008M
KFe(CN)6.H2O. The absorbance was measured at 395nm wavelength within 10 minutes. Tannic acid was
used as reference to establish the calibration curve.
Phytic acid determination was carried out according to the method described by Makkar et al., (2007) [13].
The method depends on an iron phosphorus ratio of 4:6 and is based on the ability of standard ferric chloride
to precipitate phytate in dilute HCI extract of the sample. Five grammes (5g) of the sample was extracted
with twenty milliliters (20ml) of 3% trichloroacetic acid and filtered. Five milliliters (5ml) of the filtrate
was used for the analysis; the phytate was precipitated as ferric phytate and converted to ferric hydroxide
and soluble sodium phytate by adding five milliliters (5ml) of IM NaOH. The precipitate was dissolved
with hot 3.2M HNO3 and the absorbance and immediately at 480nm. Preparation of standard curve for
phytic acid was done as follows: standard curve of different Fe(NO3)3 concentrations was plotted against
the corresponding absorbance of spectrophotometer to calculate the ferric iron concentration. The phytate
phosphorus was calculated from the concentration of ferric iron assuming 4:6 iron: phosphorus molar ratio.
Determination of Trypsin activity in Jatropha curcas kernel meal was carried out following the method
of Makkar et al. (2007). The trypsin inhibitor activity (TIA) was measured indirectly by inhibiting the
activity of trypsin. A synthetic substrate, benzyl-DL-arginine - para-nitroanilide (BAPNA) is subjected to
hydrolysis by trypsin to produce yellow colored p-nitroanilide. The degree of inhibition by the plant extract
to produce yellow color is a measure of TIA and is measured at 410nm using a spectrophotometer.
The determination of lectin activity was carried out according to the method described by Aregheore et al.
(1998) [14] which involved the use of latex agglutination and haemagglutination assays. The cake extract was prepared using 0.9% (w/v) NaCl solution mixed with an equal volume of latex bead in a round bottomed
well of microtitre plate. The plates were gently shaken at room temperature in a shaker incubator for 3 to 4
hours to obtain a homogenous solution. For latex agglutination assay, agglutination of the bead was seen as a
uniform circular clump at the bottom of the well, while a negative pattern (indicating no agglutination) was
a suspended form similar to that of blank. For haemagglutination assay, plates were left at room temperature
for 1 to 2 hours and read. A positive pattern which indicated agglutination was a uniform coating of the
bottom of the well by erythrocytes while a negative pattern (indicating no agglutination) was a circular
clump of erythrocytes surrounded by a concentric clear zone of equal size to the blank. For both the assay,
lectin activity is expressed as reciprocal of the minimum quantity (in mg) of J. curcas L. seed cake/mL of the
assay which produced agglutination.
Statistical Analysis
The data obtained were subjected to one way analysis of variance (ANOVA) and the mean value were
compared with Duncan posthoc test (p<0.05) using SPSS 19.0 software.
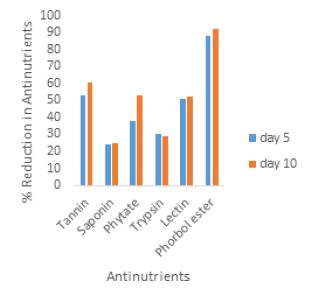
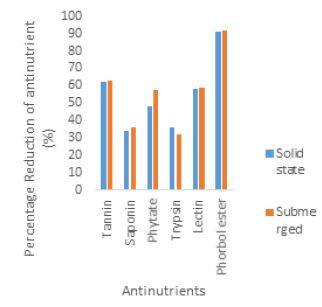
Result and Discussion
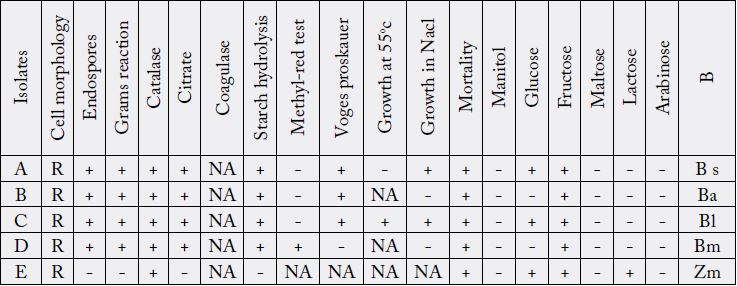
Key: NA-Not applicable, + positive, - negative, B - Bateria, BS-Bacillus subtilis, Ba-Bacillus alvevi, Bl-Bacillus licheniformis, Bm-Bacillus megaterium, Zm-Zymomonas mobilis. R - Rod.
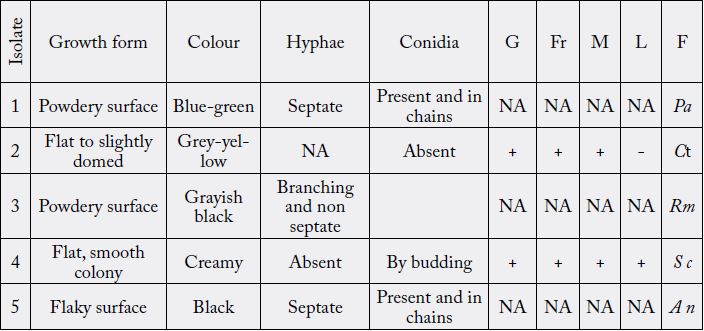
Key: NA-Not applicable, + positive, - negative, G-Glucose Fr-Fructose, M-Maltose, L-Lactose F- Fungi Pa- Penicillium album, Ct-Candida tropicalis, Rm-Rhizopus microsporum, Sc-Saccharomyces cerevisiae, An- Aspergillus niger
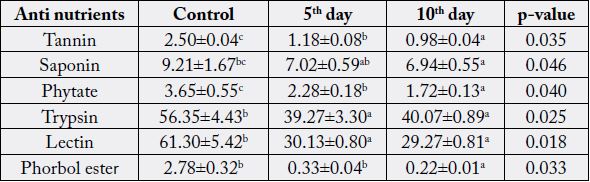
Values are Mean±Standard Error of Mean of duplicate determinations. Values with different superscripts along the column are significantly (p < 0.05) different.
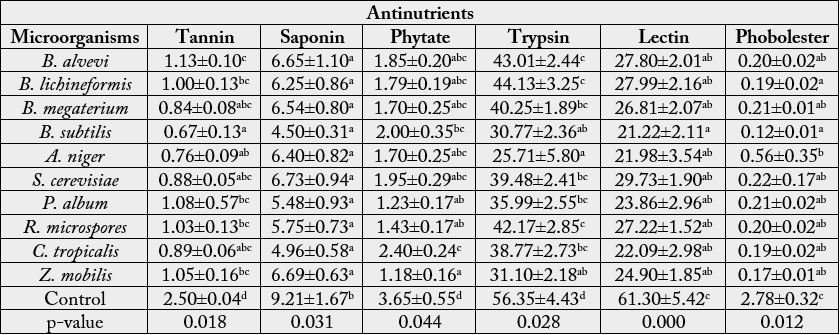
Values are Mean±Standard Error of Mean of duplicate determinations. Values with different superscripts along the column are significantly (p < 0.05) different. p-values less than 0.05 also indicates a significant difference within the treatment groups along the column.
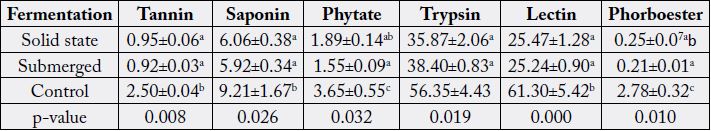
Values are Mean±Standard Error of Mean of duplicate determinations. Values with different superscripts along the column are significantly (p < 0.05) different. p-values less than 0.05 also indicates a significant difference within the treatment groups along the column.
Discussion
Fermentation processes play important roles in food technology. These processes add to the nutritive values
of foods as well as enhance flavor and other desirable qualities associated with digestibility and edibility [15].
Fermentation can produce important nutrients or eliminate anti-nutrients. The duration of fermentation
have a positive or negative influence on the nutritional values, anti-nutritional factors and toxin of food.
This may be as a result of adaptation of microbial cells to their new environment or accumulation of toxic
substances associated with by-products [16]. The result obtained from this study revealed that the longer
the fermentation time, the lower the levels of tannin, phytate and phorbol ester in Jatropha curcas kernel
meal (JCKM) when compared with the control (Table 4). The duration of fermentation did not affect the
levels of trypsin, lectin, saponin in JCKM. This findings were not in accordance with the result of Oladele
and Oshodi (2008) [17], who observed increase in phytate and tannin levels in fermented J. curcas kernel
meal on the effect of fermentation on some chemical and nutritive properties of berlandier nettle spurge
(Jatropha cathartica) and physic nut (Jatropha curcas) seeds. The significant reduction in tannin, phytic acid and
phorbol ester levels during this period of fermentation, could be as a result of adaptation of microorganisms
to their environment and production of various enzymes (including cellulase, xylanase, xylosidases, hemicellulase, amylases, beta glycosidase, proteinases, pectinases, and alphagalactosidae) during their
vegetative and reproductive phases [18]. These enzymes, secreted during fermentation period, could have
contributed to the detoxification of the Jatropha kernel meal (Jacqueline and Visser, 1996). These findings
are in conformity with the work of Belewu and Sam (2010) [19] who reported drastic reduction in phytate,
lectin, trypsin inhibitors and saponin concentrations in fermented JCKM by solid state fermentation
with the fungi Aspergillus brasiliensis, Penicillium chrysogenum, Rhizopus oligosporus, Rhizopus nigricans and
Trichoderma longibrachitum with the exception of phorbol ester that was only reduced to tolerable levels in
Aspergillus niger treated samples. Microbial detoxification can therefore be used to reduce anti-nutrients in
Jatropha kernel meals to a tolerable level using a longer period of duration (Table 3).
A time dependent antinutrient reduction (%) was observed in figure 1 and 2, during the periods of fermentation. Increase reduction of antinutrient with increase fermentation time in all the analyzed antinutrients was observed except for trypsin. Higher reduction of trypsin was observed at the end of day five when compared with the tenth day of fermentation (30% and 28% respectively). The highest percentage reduction was observed in phorbol ester determined after tenth day fermentation period. The lowest reduction was noted at the fifth day of fermentation for saponin.
The abundance of anti-nutritional factors and toxic substances in plants used as human foods and animal feeds certainly calls for concern. However, the content and quality of leguminous plants having these antinutrients can be improved to the barest minimum by fermentation [20]. Jatropha curcas kernel meal (JCKM) is a potential source of protein supplement in animal feed. However the presence of various toxic components and anti-nutrients make it unsafe for animal consumption [21]. Bacterial and yeast fermentations involving proteolytic activity are expected to increase the biological availability of essential amino acids and degrade carbohydrates and other unwanted substances. The result in this study shows significant reduction in the concentrations of all the analyzed anti-nutrients in JCKM fermented with different microorganisms when compared with the control. This agrees with the findings of Phengnuam and Suntornsuk (2014) [22], which demonstrated the removal of toxic compound and anti-nutritional factors in JCKM by bacterial and fungal fermentation thereby enabling the fermented kernel meal to retain its high protein content and other nutritional values applicable to the animal feed industry. The drastic reduction in anti-nutrients (tannin, saponins, lectin and phorbol ester) observed for Bacillus subtilis fermented JCKM may be as a result of effective breakdown and degradation of anti-nutrients by the microorganism into smaller units by the action of the enzymes (protease, phytase and esterase) mobilized during the fermentation [23]. This confirmed the earlier findings of Phengnuam and Suntornsuk (2013) and Taylor et al. (2007) [24], who reported toxins and anti-nutritional factors detoxification in the JCKM by Bacillus species (Table 4).
Fermentation is the technique of biological conversion of complex substrates into simple compounds by various microorganisms such as bacteria and fungi [25]. Fermentation has been classified into solid state fermentation (SSF) and submerged fermentation (SMF) mainly based on the type of substrate used during fermentation. In the solid state fermentation technique, the substrates are utilized very slowly and steadily, so the same substrate can be used for long fermentation periods. Hence, this process supports controlled release of nutrients. Solid state fermentation is best suited for fermentation techniques involving microorganisms that require less moisture content. However, it cannot be used in fermentation processes involving organisms that require high aw (water activity), such as bacteria. [26]. While submerged fermentation technique is best suited for microorganisms such as bacteria that require high water activity (aw). An additional advantage of this method is that purification of products is easier (Gupta and Kar, 2009) [27]. In the present study, there was significant reduction (p<0.05) in tannin, saponin, phytate, trypsin, lectin and phorbol ester levels by fermentation processes when compared with the control. This is in accordance with the findings of Belewu et al. (2009) [6], who reported drastic reduction of anti-nutritional factors in JCKM after seven days solid state fermentation with microorganisms and Sawang-arom and Suntornsuk (2011) [23], who reported significantly the elimination of toxins with Rhizopus oligosporus by approximately 36% within seven days of submerged fermentation. Neither of the fermentation processes (solid state and submerged) affected the levels of tannin, trypsin, lectin and saponin content to any appreciable degree. Although reduction in phytate and phorbol ester levels by submerged fermentation (SMF) occurred but this reduction was not significant when compared with that of solid state fermentation (SSF). These observations are however contrary to the reports of Phengnuam and Suntornsuk (2013) [28] as they observed better biodegradation of toxin and anti-nutrients in submerged fermented JCKM when compared with solid state fermented JCKM (Table 5) [29].
Conclusion
Jatropha curcas seed cake is a potential source of protein supplement in animal feed and its analysis have revealed the presence of lectins, trypsin inhibitors, saponins, lectin , tannins and phorbol ester as anti nutritional factors and toxins that can be eliminated through microbial fermentation. Detoxification of jatropha curcas kernel meal with B. subtilis using submerged fermentation have shown to be a promising method for minimizing the anti nutritional factors and toxins to tolerable level if not completely eliminated. The result from this study showed that submerged and solid state fermented Jatropha curcas kernel cake (JCKM) by Bacillus subtilis had almost similar degree of detoxification when compared with the control (unfermented JCKM). Both solid state and submerged fermentation had similar detoxification effect and proved to be the most effective method for reducing the anti-nutritional factors and phorbol esters to tolerable levels. Detoxified Jatropha curcas kernel meal can be use as an additional and or alternative source of feedstuff for livestock animals either by submerged or solid state fermentation process.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!