Biography
Interests
Shatzmiller Shimon
Department of Biological Chemistry, Ariel University, Ariel, Israel
*Correspondence to: Dr. Shatzmiller Shimon, Department of Biological Chemistry, Ariel University, Ariel, Israel.
Copyright © 2019 Dr. Shatzmiller Shimon. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Significant dysbiosis occurs in the bowel microbiology of stroke patients. The thickening of these extensive and complex changes in one index will significantly help the clinical use of microbial data in the gut. Scientists developed a microbiota in the intestines in patients with acute ischemic stroke based on microbial colon patterns, and tested whether the index was correlated with brain injury and early outcome [1].
Dysbiosis of the gut microbiota has recently been essential for the development of a variety of chronic inflammatory diseases, such as obesity, type 2 diabetes, and non-alcoholic fatty liver disease. Bacteria make phosphatidylcholine and l-carnitine from nutrients to trimethylamine, which is absorbed and further oxygenated by the hepatic flavin monooxygenase to trimethylamine-N-oxide (TMAO) [2], a metabolite that can promote partial atherogenesis by promoting the formation of foam cells Macrophages. The blood TMAO [3] level was reported to be positively associated with the risk of long-term mortality in patients with atherosclerosis, heart failure, and chronic kidney disease. In addition to the trimethylamine route, metagenomic research has suggested that symptomatic atherosclerosis is associated with altered bowel microbial 8. These results suggest potentially essential roles for gut microbiota in cardio and cerebrovascular diseases including transient ischemic stroke attack (Trans Ischemic Attack, (TIA). Such an attack is often called a mini- stroke, but it is really a great warning. TIA is a temporary blockage of blood flow to the brain. Since it does not cause permanent damage, it is often ignored. But that's a big mistake. TIAs may indicate a full stroke forward ץ When you first notice the symptoms, get immediate help. See a detailed illustration of TIA. However, these diseases have rarely been tested relative to the TMAO level and microbiota in the intestines.
Microbial research, especially of the intestines, has recently received much attention in the medical field Research is due to technological advances in metagenomics and metabolomics. Despite that, a lot Of the study's direction has focused on long-term or chronic effects of microbiota manipulation Health and disease. In this appendix, we reflect our latest publication that reported findings With reference to an alternative hypothesis. Bacterial pneumonia is prevalent and is one Of the leading contributors to morbidity and stroke. However, microbiological Cultures of samples taken from a stroke patient with a suspicious case of pneumonia often return with Negative result. Therefore, we suggested that infection after stroke can be due to the presence of Anaerobic bacteria, possibly those originating from the host microbiota host. This support, we Showed that stroke promotes bowel barrier collapse and substantial microbiota changes, and The subsequent translocation of the bacterial strain selectively from the host microbiota to the peripheral Tissue (ie lung) causes infections after stroke. Our findings were further supported by various factors Elegant studies published in the last 12 months. Here, we discuss and provide an overview of our Key findings, supporting studies and implications for future progress in stroke research.
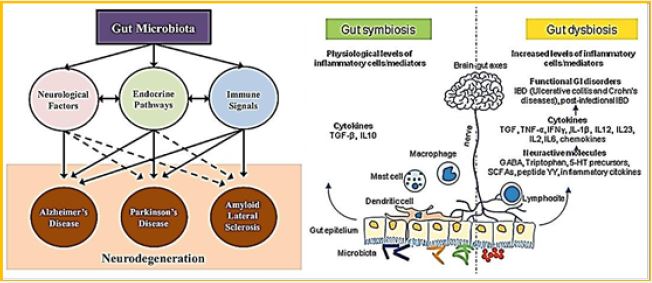
The intestinal microbiome has been extensively studied for its biological diversity and its potential role in diseases that affect the outside of the Gastro-intestinal (GI) tract [5,6]. Recently, a lot of effort has focused on understanding the intestinal-brain axis and the external communication between the GI system and the nervous system. An intensive effort of research has also revealed the involvement of intestinal microbial in improving the degree of neurological disorders, including neurodegenerative diseases. The researchers found that various microbial and dysbiosis profiles exist in patients with Alzheimer's disease, Parkinson's disease, multiple sclerosis and multiple sclerosis. In various approaches to re- establish the balance of intestinal microbial, from antibiotic treatment, fecal microbiota, or ingestion of psycho-biotics, we will discuss this review in the specific context of the struggle against degenerative diseases. Current studies and clinical trials indicate that although there is considerable potential for microbial change in the gut to be preventative or therapeutic, there are still many components of the gut-brain axis in the game, so further research is needed to define microbial factors that may alleviate symptoms of cerebral degeneration.
The gut microbiota is essential for health and has recently become a live target for bacterial biotherapies for various chronic diseases, including metabolic syndrome, diabetes, obesity and degenerative diseases. Probiotics Biotherapies are known to create a healthy intestinal environment by balancing bacterial populations and promoting their comfortable metabolic activity. The microbiota and corresponding metabolites communicate to the host using a series of biochemical and functional links thus affecting host hostage and health. Specifically, the gastrointestinal tract connects with the central nervous system through the brain-brain axis to support neuronal development and maintenance while intestinal dysbiosis is manifested in neurological diseases. There area few (three) fundamental mechanisms that regulate the connection between the intestines and the brain: direct neuronal communication, endocrine signaling mediators, and the immune system. Together, these systems create a highly integrated molecular communication network to link systemic imbalances with the development of neurodegeneration including insulin regulation, fat metabolism, antioxidant markers and immune signaling. Age is a common factor in the development of neurodegenerative diseases and probiotics that prevent many harmful effects of aging, such as decreased levels of the neurotransmitter, chronic inflammation, oxidative stress and apoptosis - all of the factors that have been shown to relieve neurodegenerative disease. Indeed, patients with Parkinson's disease and Alzheimer's disease have a high rate of gastrointestinal disease, and is suggested by some of the managers of the colon can prevent or alleviate the symptoms of these chronic diseases.
The effect of gut macrobiotics on the brain is profound and recognized to influence behavior (anxiety, depression, learning and memory, sociability), microbial activity, BBB integrity, neurogenesis and neurotransmitter production. Recently, it has been realized that brain injury and various psychological conditions can also affect the composition of the gut microbiota and possibly accelerate the disease. For example, brain injury in the form of stroke was shown to alter the composition of the microbiota in mice with specific changes in Peptococcaceae and Prevotellaceae, correlated to the extent of injury [7].
Microbiota of intestines is critical to health with changes associated with diverse human diseases. Studies have shown that altering microbiota in the intestines can significantly affect brain function. However, if the brain function change directly affects the microbiota is unknown [8]. Since it is not clear at this time how a brain injury results in clinical complications such as paralyzed infections or ileus, significant contributors to long-term hospitalization and death after stroke, we tested the hypothesis that brain damage induced changes in the gut microbiota. An experimental stroke changed the composition of the macular microbial, with specific changes in Peptococcaceae and Prevotellaceae correlated with the extent of the injury. These effects are mediated by the release of noradrenaline from the autonomic nervous system with a change in cecal mucoprotein production and cell cup numbers. Traumatic brain injury also caused alterations in the gut microbial, confirming brain injury. Changes in the gut microbiota after brain injury may affect recovery and treatment and patients should evaluate such changes [9].
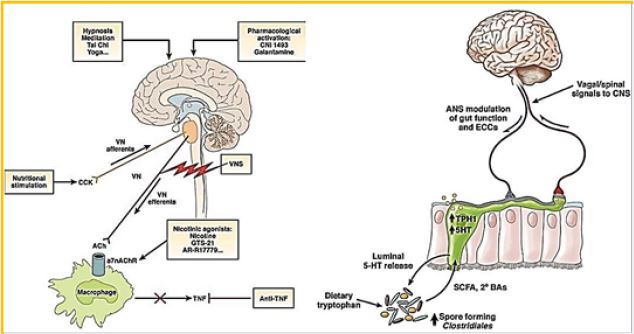
Clinical and pre-clinical studies have shown bi-directional interactions on the brain-gut- microbial axis [7,11]. Bacterial bacteria communicate with the CNS using at least 3 parallel channels of interaction involving nerves, endocrine, immune signaling mechanisms. The brain can influence the structure of the community and the functioning of the microbiota in the intestines through the autonomic nervous system by altering the bowel movements, intestinal secretion and intestinal permeability, and potentially by luminal secretion of hormones that directly regulate the expression of the bacterial gene. A biological model of systems is proposed, which places circular communication loops between the brain, intestines and colon, during which the disturbance at each level can spread dysregulation along the circuit. A series of preclinical observations mainly affect changes in the brain-gut- microbiome in the pathogenesis and pathophysiology of irritable bowel syndrome, obesity, and some psychiatric and neurological disorders. Ongoing research fulfills the promise of identifying novel therapeutic targets and developing therapeutic strategies to address some of the most debilitating, costly, and severely debilitating diseases.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!