Biography
Interests
José López Castro1*, Ángel Alfredo Martínez-Ques2, María Concepción Martín-Arribas3, José Rumbo Prieto4, María Veiga-Rodríguez5, Miriam Vázquez-Campo6, Beatriz Braña-Marcos7, Laura Herrero Olivera8 & Juan Gómez-Salgado9
1MD. PhD. Internal Medicine Dep., Hospital Monforte de Lemos (EOXI Lugo), España
2PhD, RN. PAC Castro Caldelas (EOXI Ourense), España
3PhD. Instituto de Salud Carlos III, España
4PhD, MS, RN. Quality Unit: Care, Research and Innovation, Complejo Hospitalario Universitario de Ferrol, España
5PhD, MS, RN. Hospital Quirónsalud A Coruña, España
6PhD, MS, RN. Nursing Dep., Universidad de Vigo, España
7PhD, MS, RN. General Direction of Care Planning, Consejería de Salud del Principado de Asturias, España
8PhD. Dep., Theory of Knowledge and History of Thought, Faculty of Philosophy, Universidad Complutense, Madrid
9PhD, MS, RN. Nursing Dep., Universidad de Huelva, Huelva (España)
*Correspondence to: Dr. José López Castro, Internal Medicine Dep., Hospital Monforte de Lemos (EOXI Lugo), España.
Copyright © 2019 Dr. G. M. José López Castro, et al.. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
An important aspect of the quality of care at the end of life, that the care provided congruent with the wishes of the patient, so the main objective in this study is to show the elaboration metodology to develop an instrument to measure the quality of the process of advance care planning. In this sense, it was planned a descriptive observational, multicenter study of validation of a questionnaire, in Hospital Centers (CH) and Primary Care (PC) of the Public Health System of Norwest of Spain, with an expected duration of 3 years. Sample for validation through random assignment by conglomerates in two stages. This article describes the methodology applied.
Introduction
An important aspect of the quality of care at the end of life is that the care provided is consistent with the
wishes of the patient. The temporary or permanent decrease in the patient’s capacity can make good quality
health care difficult, if their preferences for this situation are unknown. Patients and healthcare professionals
can foresee and anticipate these future situations and make decisions on how to proceed in the event of
loss or lack of capacity. This can avoid unnecessary and unwanted care, increase patient autonomy and
satisfaction and improve the quality of care [1].
One way to adapt the wishes and values of each patient to future care is through their participation in advance care planning (ACP). The ACP is a consequence of a new style of patient-centered clinical relationship, which understands healthcare as a non-occasional, sporadic or fortuitous, but structured continuum, where patient preferences and their decision-making autonomy are really taken into account [2]. The number of people registered (with data from January 2019) in the National Registry of Prior Instructions in Spain, was 305.290, which represents a rate of 6,53 per 1,000 inhabitants, an amount well below what would be desirable.
The approach of ACP in Europe is uneven. There are countries that have regulated advance directives (Germany, Austria, Belgium, Spain, Finland, France, Netherlands, United Kingdom) and other countries that lack specific legislation (Bulgaria, Greece, Italy, Norway). Nor is the regulation nor its binding force uniform. The ACP approach has developed more strongly in countries with prior regulation of the PI such as Germany, Belgium, the Netherlands and the United Kingdom [3].
The ACP has advantages for the patient, because it ensures that his will is fulfilled at the moment when the situation comes when he is not able to express it personally, and it is also a great help for the professional to clarify the care that can be applied when he has to take care of the patient. In the clinical practice, it has been associated with positive results of improving the quality of care, including the reduction of the risk of dying in the hospital, greater use of palliative care, greater concordance between the desired care and cost reduction [4-6].
Despite the advantages and clinical evidence, the implementation of ACP in clinical practice is limited. Regarding the design and validation of ACP process measurement questionnaires, the pioneering works Nolan et al [7] stand out with the 17-item The Advance Directive Attitude Survey, Sudore et al. designed a questionnaire to detect a change in behavior in the ACP [8] and it should be mentioned as an option that the Respecting Choices® initiative [9] is proving very promising. In Spain, observational studies predominate over experimental ones, which are focused on the measurement of attitudes and knowledge about ACP in professionals and patients. Despite the lack of knowledge, professionals show favorable attitudes towards the use, utility and IP document [10-12].
Given the lack of studies that measure the scope of the ACP, a validated measuring instrument is needed that evaluates the entire range of processes involved in the ACP.
Methodology
Its main objective is to elaborate and validate an instrument for measuring the quality of the process of ACP
as well as determining the influence of certain external factors (clinical environment) that favor the advance
planning of decisions. In addition, the clini-metric properties of the questionnaire should be determined:
Establish the reliability of the questionnaire, analyze the intra-observer concordance for each of the items
in the questionnaire, determine the internal consistency of each of the items in the questionnaire, assess
the stability of the questionnaire and sensitivity to change, verify the validity (of content and construct) of
the questionnaire, establish the existence of relationships between external factors and the reliability of the
questionnaire, and study which dimensions of the questionnaire best predict the intention of a patient’s
behavior to the perform and registration of a document of PI. A descriptive, multicentric observational study
of the validation of a questionnaire in Hospital Centers (CH) and Primary Care (AP) of the Public Health
System of the Northwest of Spain of 3 years has been chosen as a statistical design.
Two types of samples will be considered as shown in Table 1. For the calculation of the sample size necessary to validate the measuring instruments an estimate will be made based on the calculation of Cronbach’s α. The final calculation will be carried out once the definitive number of items of the questionnaire is known once it has been designed and passed the judges test, assuming a level of significance of 95%, a statistical power of 80% and a Cronbach’s α value for the null hypothesis contrast equal to 0.6, and to detect A Cronbach’s alpha equal to or greater than 0.7. The type of sampling will be by random assignment by conglomerates in two stages and the Health Centers and services of Hospital Consultations of 4 Clinical Areas of the Northwest of Spain will be randomized, chosen by a random sequence generated by simple randomization, with statistical program Epidat 3.1. Patients in the selected units will be recruited by consecutive sampling. For data collection, the instruments will be those designed and built by the research team:
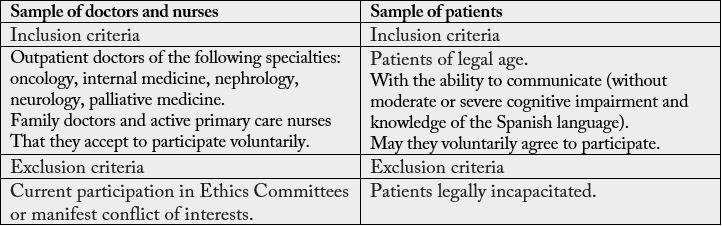
PROACP instrument designed by researchers, will consist of two parts:
- Professional scale with socio-professional data and dimensions of the ACP perceived by the professional. The final dimensions will be made with the study carried out by the researchers.
- Patient scale: with socio-demographic data and dimensions of the ACP that the patient perceives. The final dimensions will be made with the study carried out by the researchers.
Process of Recruitment and Recruitment of Professionals: A member of the research team will contact (by phone, email or letter) with the coordinator or head (doctor and nurse) of each unit / center selected in the sample of the study, proceeding to inform the objectives of the study and request their collaboration to arrange a meeting with the health professionals of each unit / center. Once the meeting appointment has been arranged, the contracted support staff (health professional linked to the Health Service) will be the one who will go to the center on the days and hours previously agreed. The recruitment to the study will be carried out by voluntary participation of each professional, for which they must have previously signed the informed consent. Participating professionals will be provided, by the support staff, with a coded selfcompletion questionnaire (with the study variables), which will be collected, after completion, by the support staff or deposited in a cardboard box / mailbox closed, if they wish. In the case of the sample selected for the pilot study, the agreed day to go to the center, following the request for consent and the delivery of the questionnaire, the contracted support staff (health professional linked to the Health Service) will arrange a new appointment with the selected staff within one week for the test-retest. The following variables must be collected:
- Main variable. Score obtained through the PROACP Questionnaire.
- Secondary Variables:
a) Sample of patients: Socio-demographic of the population studied: age, gender, civil status, main medical
diagnosis, population, socioeconomic level, level of studies, Chronic disease, granting of previous instructions,
preference of person to deal with on the subject, preference on the contents of the previous instructions,
own perception of health status and the functional dependence (people 65 and older).
b) Sample of professionals: Socio-professionals: age, gender, profession, healthcare level, years of professional
experience, years of experience in patients with cancer or palliative care, academic level, province, institution,
center, unity, number of patients treated on the last day of consultation, hours for consultation on the last day.
10 independent national experts of different geographical distribution will be selected to guarantee the independence of their decisions. The selection will be intentional based on the following attributes:
- Clinical experience of more than 10 years, preferably in the Oncology, Neurology, Nephrology, Internal Medicine / Palliative and Primary Care Department, as these services where ethical issues are most frequently raised at the end of life.
- Advanced knowledge in research methodology and / or validation of questionnaires.
- Experts in Bioethics
The participation of the experts will be done via electronic survey using the tool available in Google Docs and alternatively by email, guaranteeing at all times the anonymity of the participants in the Delphi technique during the development of the same.
The instruments will be piloted to assess the comprehension, acceptability and completion time of the
questionnaire. The selection will be intentional, to facilitate its subsequent location again in order to carry
out the test-retest one week apart.
In the first place, the interview will be conducted with the professionals and then with the patients. Prior to
the interview, informed consent will be sought, once the objectives of the study have been explained, they
will be encouraged to answer sincerely. The collected data will be entered into a database created for this
purpose with an Excel table.
Statistical analysis: Statistical analyzes will be carried out through the statistical program Statistical Package for the Social Sciences (SPSS 21). A descriptive analysis of the sample of patients and professionals will be carried out using frequency and percentages for qualitative variables and central dispersion measures for quantitative variables.
For the psychometric analysis:
Content validation of judges: Kendall’s non-parametric concordance test to prove the agreement of the experts.
Quantitative validation: Psychometric analysis of the properties of the scale:
Reliability: Measures variation or homogeneity in measurements: Cronbach’s alpha coefficient will be used.
Internal consistency of the questionnaire, or correlation between the items of a dimension: through the correlation of Pearson, Spearman or Kuder - Richardson as appropriate.
Discriminant power: Pearson or Spearman correlation coefficient.
Intra-observer or test-retest reliability: Intraclass correlation coefficient for quantitative variables and Sperman- Brown correlation for ordinal qualitative variables.
Content Validity: Exploratory factor analysis.
The Barlett sphericity test and the Kaiser-Meyer-Olkin (KMO) test that is considered satisfactory for values greater than 0.7 will be used. Both tests are carried out to verify that the inter-correlation matrix between the items is appropriate for the performance of the factor analysis. Subsequently, the total variance explained by each factor will be calculated and the factors will be extracted, according to the Kaiser-Guttman standardization criteria. A matrix of weighted factors will be created and to simplify its structure, several procedures will be carried out: factor analysis with the “promax” method, next, factor analysis with the “equamax” method, and finally, a refactoring will be carried out with the “varimax” method. Finally, the correlation between each of the factors identified with the Pearson coefficient (r) will be calculated, thus creating a correlation table or matrix.
Analysis of the influence of sociodemographic, socio-professional and clinical environment on reliability (Chi-square, t Student). Significance with p value <0.05, confidence intervals at 95.
Ethical and Legal Aspects
This study has obtained the favorable report of the Research Ethics Committee of Pontevedra-Vigo-Ourense
(Spain), with the Registration Code: 2017/158.
Funding
Project financed by the Health Research Projects (in the Research Projects modality) of the 2016 call for
the Strategic Health Action 2013-2016 in Health and co-financed by the European Regional Development
Fund (ERDF). No. exp: PI16 / 01686.
Conflict of Interests
There are no conflicts of interest.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!