Biography
Interests
Haixia Wang1, Yanhong Gao2 & Jin Gao1*
1Department of Pharmaceutical Sciences, College of Pharmacy and Pharmaceutical Sciences, Washington State
University, Spokane, WA 99202, USA
2Shangqiu Municipal First Senior High School, Shangqiu, Henan 476000, RPC
H. Wang and Y. Gao contribute equally to this work
*Correspondence to: Dr. Jin Gao, Department of Pharmaceutical Sciences, College of Pharmacy and Pharmaceutical Sciences, Washington State University, Spokane, WA 99202, USA.
Copyright © 2019 Dr. Jin Gao, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Both eukaryotic and prokaryotic cells secrete extracellular vesicles (EVs). To meet the requirements of clinical application, the facile EVs were also prepared by various physical, chemical and biological methods to mimic the natural ones. Although some EVs have been at the phases of clinical trials, there are lots of challenges facing the clinical application of EVs. Herein, the most outstanding problems concerning the three stages of commercialization of EVs, including production scalability, quality control and immunotoxicity, were comprehensively discussed. In addition, some strategies toward solving these issues are also proposed.
Introduction
According to concept of ISEV (The international Society for Extracellular Vesicles), “extracellular vesicles
(EVs) are defined as the particles naturally released from cells that are comprised of a lipid bilayer membrane [1]. Acting as important mediators between cells that regulate both physiological and
pathological conditions in the living bodies, EVs are nanosized spherical compartments and contain lipids,
proteins and various nucleic acids of their source cells [2,3]. Based on their biogenesis and sizes, EVs are
generally categorized into three types, including exosomes, microvesicles and apoptotic bodies. Exosomes
are generated from endosomal compartments in the cells. The size of exosomes is around 30 to 100nm
in diameter. Microvesicles are directly produced through outward budding and fission of the cell plasma
membrane, and their sizes have a wide range between 50-2000nm. When cells are going to die or subject
to apoptosis, the cell membrane is disrupted to form apoptotic bodies with the size of 50 to 5000nm [4,5].
Besides EVs derived from eukaryotes, prokaryotes also secrete EVs. It has been reported that Gram-positive and Gram-negative bacteria can both generate EVs [6-8]. Gram-negative bacteria spontaneously release EVs via shedding from outer membrane, so-called outer membrane vesicles (OMVs). Since Gram-positive bacteria do not have “outer membrane” when compared to Gram-negative bacteria, Gram-positive bacteria release OMVs from the inner membrane and the released membrane vesicles go through the cell wall and form so-called OMVs. The size of EVs from Gram-positive bacteria was reported to be ~20-100nm in diameter, which was similar to EVs derived from Gram-negative bacteria [8].
Inspired by the generation of naturally secreted EVs, many researchers are also seeking to prepare biomimetic EVs by physical [9,10], and chemical [11] methods in recent years. In general, the artificial EVs exhibit similar properties to the natural ones. As a supplementary to the natural EVs, biomimetic EVs possess some advantages in some aspects, such as purity, yield, targeting ability, etc.
There is no doubt that the purpose of all above efforts is to translate EVs and utilized their good properties to serve the humankind’s healthcare. Though some application of EVs have been tried in animals, there are only few cases for their clinical use [12-14]. In this review, the most outstanding challenges in each stage of commercialization of EVs will be reviewed and the potential solving direction will also be discussed.
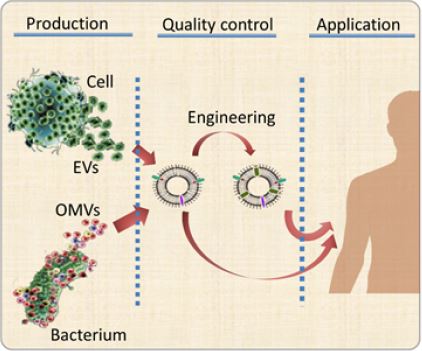
Challenges in Application
Although some clinical trials on the applications of EVs have been reported, there are still some challenges
existing in the three necessary stages of commercialization, namely production, quality control and application
(Figure 1). Here, three most remarkable problems of each steps will be specified.
First of all, it is necessary and urgent to develop a reproducible and scalable approaches to generate clinic-grade
EVs [15]. Some strategies have been applied to improve the generation of natural EV, for example culture
conditions, such as hypoxia [16], increased calcium concentrations in medium [17,18] and serum starvation
[19] may increase the production of EVs. In addition, the continuous culture system has shown the high
yield of EVs by 40 times compared to traditional culture flask settings [20]. To scale up the manufacturing
process of EVs, a new method have been developed based on nitrogen cavitation to generate biomimetic
nanovesicles, and the result showed that the production of EVs was 16-fold higher than naturally secreted
EVs [21]. In addition, Gho et al. [22,23] also utilized extrusion and sonication to achieve the very high
production of EVs by over 100 folds compared to natural secreting way. Although it looks like that the selfassembly
of phospholipid and membrane proteins can form EVs-like nanoparticles [24] and their scalability
is possible, this self-assembly process cannot guarantee the surface orientation of membrane proteins and
isolated membrane proteins may not maintain conformations after they leave the cell membrane. To meet
the basic requirements of biopharmaceuticals (such as repeatability, efficiency and costs), the bioreactor
system [20] may hold the most promising perspectives in manufacture of EVs. Although the generating
method is a little bit cost- and labor-consuming, the method can produce EVs efficiently in a continuous
way. However, the success of this method is dependent on the physiological conditions, including aging, of
cell culture.
Besides the necessary bioactive and targeting ingredients, there are also a lot of components in EVs, including
DNA, RNA, proteins, carbohydrates, phospholipids, etc. These components can be affected by a lot of
factors, including culture conditions, physiological status (as mentioned above), and manufacturing methods.
However, there was rare reports regarding the difference between EVs generated from different methods.
We once compared the components of naturally secreted EVs and artificial EVs by nitrogen cavitation, and
some differences were found [21]. However, as a biopharmaceutical, or its carrier, the uniformity is critical
for translation and an ideal method for this purpose is still lacking. The types and contents of each protein
on EVs may be characterized by proteomics way, while types and contents of each phospholipid may be
analyzed by phospholipids fatty acid spectrum.
The last concern about the clinical application of EVs is the possible immunotoxicity induced by EVs. In
the previous studies, we found the EVs generated from nitrogen cavitation can activate HUVECs, while the
function can be mitigated by the loaded anti-inflammatory drug. The following studies also demonstrate
that the immunotoxicity may be caused by the exposed phosphatidylserine, originally locating on the inner
surface of cell membrane, due to the external forces [21]. To avoid the impairment from immunotoxicity,
anti-inflammatory therapy may be necessary and helpful in some cases.
Conclusion
In the past decades, the research on EVs indicates that EVs are transforming the traditional nanoparticlebased
drug delivery because EVs have the unique features. EVs have the excellent biocompatibility, long
circulation time and low immunogenicity, and most importantly, they maintain their parent cell features that
make them excellent drug carriers. Different to the conventional drug carrier, some EVs themselves perform
as bioactive drug for disease therapy. Some special tissues, such as brain, can also be targeted by EVs, and no
special targeting ligands are required. The above features confer EVs some outstanding advantages to be a
drug carrier or disease treating agent.
In summary, this review summarized the current most eminent challenges facing each step of commercialization of EVs. With advances in nanotechnology and immunology and genetic engineering, industrialization of EVs may become more and more realistic and eventually realize, and EVs will eventually turn to be a powerful tool for therapy of many human diseases.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!