Biography
Interests
Arafa, M. I.1*, Ahmed, S. M.2, Tawfik, M. S.3 & Hefnawy, Y. A.4
1Assiut Lab, Dept. of Parasitology, Animal Health Research Institute (AHRI), ARC, Egypt
2Food Hygiene Department, Animal Health Research Institute, El-Minia Lab, Egypt
3Food Hygiene Department, Animal Health Research Institute, Assiut Lab, Egypt
4Food Hygiene Department, Fac. of Vet. Med. Assiut Univ., Egypt
*Correspondence to: Dr. Arafa, M. I., Assiut Lab, Dept. of Parasitology, Animal Health Research Institute (AHRI), ARC, Egypt.
Copyright © 2019 Dr. Arafa, M. I., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Toxoplasma gondii, is a meat cyst-forming protozoa, has a potential zoonotic impact sequence to consumption of undercooked meat or chickens harboring bradyzoites. The present study, explores some common meat and chicken products (300 samples), which are still neglected as a possible source of human toxoplasmosis. Samples of minced meat, sausage, kofta, beef burger, chicken burger and chicken kofta were examined for the persistence of viable T. gondii bradyzoites through direct microscopical examination of digested meat samples confirmed by PCR for recognition of T. gondii DNA. Mice viability testing was adopted for positive samples detected the potential zoonotic hazard. Microscopically, chicken kofta, beef minced meat followed by sausage showed the highest percentage of Toxoplasma contamination as 24, 16 & 6.7% respectively, while molecular study using PCR detected that beef minced meat, chicken burger, sausage followed by chicken kofta had 40, 20, 9.5 & 6.25% contamination percentage respectively. Otherwise, viability assay using mice passage, showed positive result only in beef minced meat (14.3%), while sausage, kofta, beef burger, chicken burger and chicken kofta were negative for viability test. The study concluded that despite of Toxoplasma contamination of different tested meat or poultry samples using different examination tools, the viable parasites were detected only beef minced meat which leads to great zoonotic public hazard on human consumption.
Introduction
Toxoplasmosis is one of the major causes of human life-threatening infection especially for high-risk
groups such as the pregnant women and immuno-compromised patients [1]. Beef products and poultry
meat products are commonly consumed foods in Egypt. Toxoplasmosis is widespread disease in humans
and many other warm-blooded animals caused by the protozoan parasite, T. gondii which is an obligate
intracellular parasite belonging to the phylum Apicomplexa [2]. Toxoplasmosis is listed as the third-biggest
cause of life-threatening food-borne infections [3], where the infective stages of the parasite can take three
different forms sporozoites, tachyzoites and bradyzoites. Following sporulation in the environment, oocysts
containing sporozoites are infective, and give rise to tachyzoites when ingested by an intermediate host
(most species of mammals and birds) [4]. Toxoplasmosis is also atypical meat borne zoonosis, where most
human infections occur through the bradyzoite stage that present in the edible meat [5]. Consumption
of undercooked meat or meat products has been regarded as the most significant risk factor of primary
infection [6] and transmission of Toxoplasma [7].
Free ranging chickens, especially in developing countries may be considered as an important source of T. gondii infection in human, particularly those kept free house ranging has a higher chance of being infected with the parasite contaminating environment. So, chicken meat products manufactured from such infected chickens may harbor T. gondii [8-11], when beunder-cooked have been associated in T. gondii outbreaks [12-13]. Large portion of human toxoplasmosis cases were thought to be derived from consumption of undercooked meat that was infected [2].
The present study aimed to studying the possibilities of some neglected meat and chicken products as a source of human toxoplasmosis.
Material and Methods
A total of 300 samples were purchased from supermarkets in Assiut and El-Minia cities. These samples
include: beef minced meat, sausage (75 samples of each), kofta, beef burger (50 samples of each), chicken
burger and chicken kofta (25 samples of each). A 100 gram of each product was used; the samples were
stored at -20ºC until examined.
Twenty gm of each sample were homogenized separately in 100ml of 2.5% pepsin in PBS and incubated
at 37°C for 1.5hr with continuous shaking. Following incubation, the digested samples were sieved through
mesh and then centrifuged for 5 min at 1,500g. Sediment was smeared on slides; make more than one smear
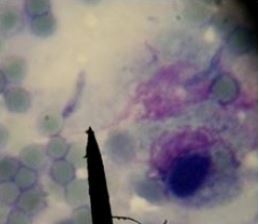
for each collected sample. Tissue smears were dried, fixed and stained by Giemsa stain. Stained tissue smears were examined at x 100 magnification under the light microscope for detection of bradyzoites [14]. PCR
assay included all positive microscopical samples as well as quite number of negative samples from all tested
products.
PCR was performed according to a recommended protocol [15]. A total of (97) samples were used for
recognition of T. gondii DNA. These samples included of 35 minced meat, 21 sausage, 10 kofta, 10 beef
burger, 5 chicken burger and 16 chicken kofta. PCR has been done in Food Analysis Center, Faculty of
Veterinary Medicin, Benha University.
The samples were minced by an electric meat grinder and 25-30mg of each minced tissue was used for the
DNA, following the manufacturer’s instructions of a commercial DNA ex “traction kit (Qiagen,Valencia,
CA, USA). The samples were resuspended in 180μL ATL buffer and 20μL proteinase K (supplied in the
QIA amp DNA Mini Kit), and the protocol recommended for tissue samples was followed. All DNA
extracts were stored at -20°C until used. This product was used as a template for PCR [16].
Application of PCR for identification of B1 gene specified for T. gondii was performed by using Primers
(Biosearch Technologies, Inc., USA) as shown in the following table 1.

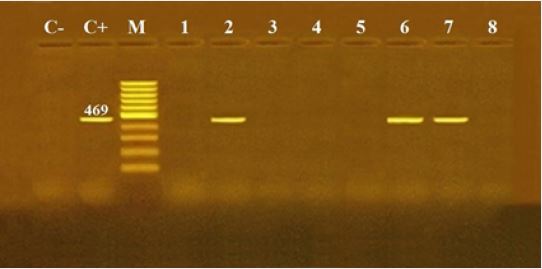
B1 gene was selected for PCR because of being highly conserved among Toxoplasma strains with 35-fold repeat gene and 2214 nucleotides in each repeat. B1 gene was targeted to generate specific primers Tg1, Tg2 and amplified 469-bp DNA fragment [17]. The PCR mixture contained 1μM of each primer (Tg1 and Tg2), 5mM of 10 x PCR Gold buffer, 1.5mM of MgC12, 1mM of deoxynucleoside triphosphate (CinnaGen), 0.3 U of Taq DNA polymerase (CinnaGen) and 20.2μ1 D.W. The reaction volume was 50μ1 containing 20 pi of DNA extracts. Reactions were preheated in thermal cycler (Bio Rad-USA) for 10 min at 95ºC, followed by 35 cycles of 94ºC for 1 min, 52ºC for 30s and 72°C for 1 min, with a final extension step at 72°C for 7 min [15]. 10μ1 of amplified products were run in 1% agarose gel [15].
Bioassay testing was designed to inoculate mice with the bradyzoites intraperitoneally according [18], all
positive PCR samples (18) were used to evaluate the viability of T. gondii, since each one of them was
inoculated into a group (3 mice) resulting in 18 group in addition to 3 mice (control group). Animal selection, care, sacrifice procedures, and the experiment was upon the instructions and guidelines for animal care of
Animal research house - Collage of medicine , Assiut University. So, 57 Swiss albino mice weighing about
25g B wt, were used. Each sample (50g) was submitted individually to peptic digestion, 1ml of each tissue
homogenate of the material resulting from the digestion was inoculated intra-peritoneal. Mice were daily
inspected for signs of febrile response that might indicate acute toxoplasmosis. Mice which showed signs
of illness were culled immediately, where peritoneal exudates was aspired and inspected for tachyzoites
existence microscopically. By the experiment terminal end (six weeks), the surviving animals were put down
and brain fragments were squeezed and examined for tissue cyst microscopically. Mice were considered
infected with T. gondii when tachyzoites or tissue cysts were demonstrable in their tissues [19].
Results
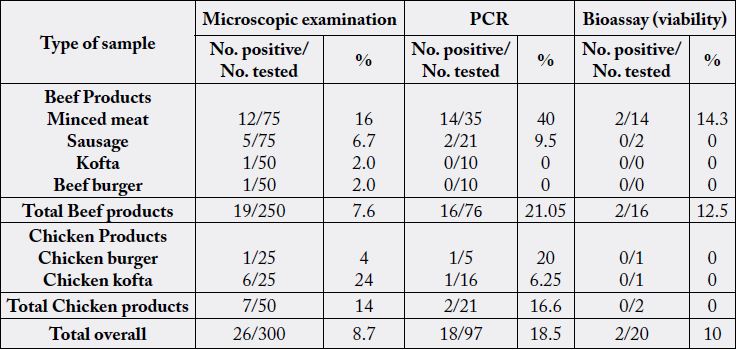
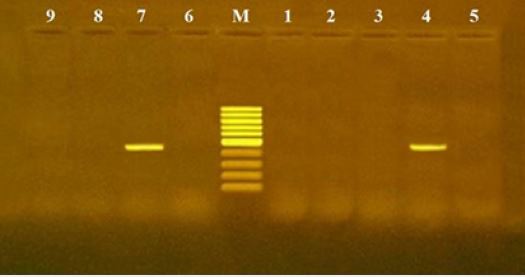
The results of microscopical examination, PCR and mice bioassay are tabulated in Table 2 and Fig. 1, 2 & 3.
Discussion
As an opportunistic pathogen, T. gondii infection rarely causes severe symptoms in healthy humans. The
main risks are congenital infection during pregnancy which leads to great social problems [20]. Latent
toxoplasmosis has also been linked to changes in cell signaling pathways that may lead to neurological
disorders including schizophrenia, epilepsy, Alzheimer’s disease and Parkinson’s disease [21-22]. The
prevalence of toxoplasmosis has been detected as higher in old animals, which are the source of meat products
as fermented sausage, salami, and others [23-24], thus become an important risk factor in the transmission
of Toxoplasma [7].
In the present work out of 300 frozen bovine and chicken meat product samples revealed that T. gondii bradyzoites infection rate was lower in beef products than in chicken products microscopically using the digestion technique. Contrarily, PCR analysis showed that beef meat was more prevalent than chicken meat (Table 2). Moreover, chicken kofta was the most prevalent infected product microscopically while minced meat was the highest using PCR technique among all products studied (Table 2) (Figure 1 & 2). The variability in microscobic and PCR results may be attributed to the heterogeneity distribution of bradyzoites in the examined sample [18]. The role of different meat producing animal species and meats thereof is controversial [25], since even in low prevalence the risk posed to consumers by ingestion of contaminated beef is likely to be high due to consumption habits [26].
According the available literatures, the present study is considered the first document to detect T. gondii in chicken products in Egypt. Many investigators reported the occurrence of T. gondii in beef products in wide variable rates as (4-65.6%) [27-28] in minced meat; [27, 29-33] in sausages; [30] and [31] in hamburger; and [32] in kofta.
PCR did not detect T. Gondii DNA either in kofta or beef burger despite of their positive microscopical results which may be attributed to salt content of these meat products which limited sensitivity of the PCR assay by inhibition of the polymerase enzyme [34].
A mouse concentration bioassay technique was used in the present study to detect the viability of T. gondii in positive PCR samples (18 samples), two samples of minced meat reflecting a frequency of 11.1% positive for viability (Figure 3). It is obvious that only the viable T. gondii cyst was detected only in raw beef minced meat, while other tested products (beef kofta, burger and sausage or chicken kofta and burger) which had much additive spices did not have any viable cyst that might be as a result of these added spices. Two home spices extracts (Piper nigrum & Curcuma longa) were studied [35] about the growth inhibition of T. gondii tachyzoites in experimentally infected mice, where it ranged (81.2 - 97.4%) in Piper nigrum and Curcuma longa respectively. Otherwise the higher positivity obtained by PCR compared with bioassay in examined samples may be because PCR results based on the B1 gene of T. gondii and not the presence of viable parasite [7].
Moreover, all tested meat or chicken products were freezing preserved which may not be low enough to kill T. gondii cysts [36]. Otherwise, the low level of isolation by the bioassay in mice could be due to the stage of development of the parasite and the size of the examined sample. Through a previous study [37], the low number of tissue cysts in infected pigs, less than 1 cyst per 50g of tissue, and the very sparse or focal distribution of parasites in the tissues are important characteristics that should be in mind when results of T. gondii are discussed concluded that a negative result from any sample does not necessarily mean that the entire tissue is free of the parasite [18]. On other hand, Meat from breeding animals is usually processed such as sausages, kofta and beef burger where processing procedures may kill or reduce T. gondii [23].
Conclusions
In conclusion overall, although T. gondii was detected in different examined meat and chicken products,
viability of T. gondii was detected only in minced meat samples. Nevertheless, consumers, especially pregnant
women, and persons with immunosuppression should be aware that they can acquire T. gondii infection
from ingestion of undercooked meat and chicken products.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!