Biography
Interests
Arafa, M. I.
Assiut Lab, Dept. of Parasitology, Animal Health Research Institute (AHRI), ARC, Egypt
*Correspondence to: Dr. Arafa, M. I., Assiut Lab, Dept. of Parasitology, Animal Health Research Institute (AHRI), ARC, Egypt.
Copyright © 2019 Dr. Arafa, M. I. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Hippobosca equina (H. equina), infest different animal species and may bite human causes many diseases transmitted among these hosts. So, the present work scanned the ultrastructure of mouthparts and adhesive device of H. equina using scanning electron microscopy. Mouth parts of adult H. equina form a tubular sucking organ sheathed within a dorsal groove of the labium which armed at the tip with short labella that serves as piercing structures of proboscis. The tip of the proboscis equipped with backward- directed teeth, around the entrance to the food-canal. At adhesive device, the pulvilli characterized by presence of numerous tenent setae observed on their ventral side which have spoon-like tip. The empodium appears as a wipe -like process has sharply pointed spins on both lateral sides. Our results provided anatomical information of mouth parts and adhesive device of H. equina that clarify their role as a mechanical carrier of microorganisms through injury or intact skin. These results strongly suggest that H. equina are not simple mechanical vector.
Introduction
Hippobosca equina (H. equina) Linnaeus, 1758, (Diptera: Hippoboscidae) are economically and medically
important ectoparasites. H. equinaare observed in Europe and named forest flies but the primary host of
this parasite is the red deer (Cervuselaphus) [1] mainly in horses [2] and cattle [3] transmitting ulcerative
lymphangitisor rumenant bortenellosis [4] also infest buffaloes transmitting odematous skin disease [5] It
also is named lice fly has zoonotic hazard on human biting [6] causing anaphylactic reactions [7] Pathogens
(bacteria, virus, protozoa cyst, helminthes ova, or larva) have been mechanically carried by flies via the
external integument, particularly organs such as the mouth parts, wings, legs, or adhesive device that come
into contact with filth or open wound [8].
The objectives of this study were to describe the morphology of the mouth parts and adhesive device of H. equine by means of scanning microscopy (SEM). This detailed anatomical description will provide new insights to the pathogens transmission process.
Material and Methods
Forty adult flies (H. eqiuna) were collected directly using forceps from infested buffaloes, cattle and donkeys
hosted together at Fayamavillage, Assuit, Egypt. They were kept into plastic sacs or wide-naked bottles for
laboratory examination where they taxonomically identified under binocular microscope according to their
morphological characters [9,10].
Adult flies were washed several times with normal saline solution, fixed in 2.5% glutaraldehyde at 4°C for
24h, then subjected to post fixation in 1% osmium tetroxide and dehydrated in a graded alcohol series. This
was followed by treatment in acetone and critical point drying. Finally, they were mounted on stubs sputtercoated
with gold and viewed under a JEOL JSM-5910LV scanning electron microscope.
Results
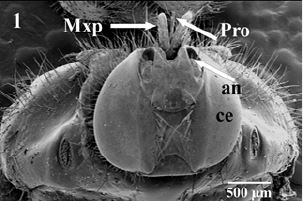
Scanning electron microscopic observations of H. eqiuna head revealed prominent organs (a pair of compound
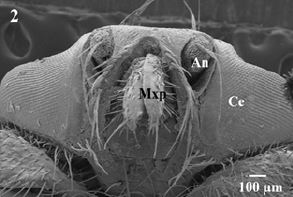
eyes, antennae, maxillary palps and a mouth parts asproboscis-Fig. 1). Theproboscis and maxillary palps are
located in a conspicuous groove surrounded by one row of long sharp sensilla (Fig. 2). Maxillary palps
areshort andun-segmented covered with few numbers of variable length slightly curved sensilla arising from
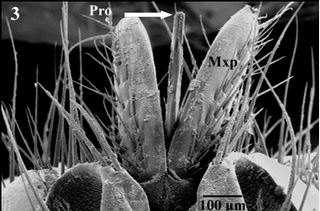
discoid socket (Fig. 3). Piercing-proboscis of H. eqiuna is about 4.8mm long which is completely covered
with maxillary palps in resting position (Fig. 2), while during feeding position it directed straightly forward
between the two maxillary palps in front of the head (Fig. 3).
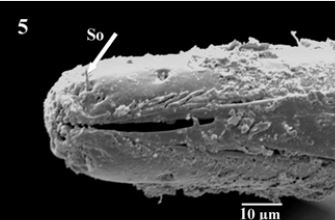
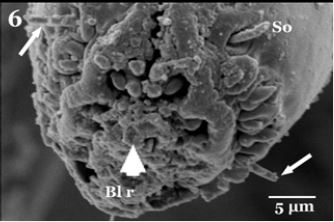
The proboscis consists of the tubular labrum (La), which forms the food canal end with rosette shaped aperture of the food canal. Labrum laterally enclosed by tubular labium (Fig. 4). Higher magnification showed presence of short labella (Lb) articulate on the apex of labium which serves as piercing structures of proboscis andlabella appeared with serrate edges bearingsensory organs on both lateral sides of the apex (Fig. 5). It is apically equipped with backward- directed teeth, around the entrance to the food-canal. The inner margin of the labella has serrate cuticular ridges, and their lobes are comparatively small, with sharpprestomal-teeth which are relatively enlarged. Scanning electron micrograph cleared completely covering the external surface of mouth parts with clotted blood residues especially aperture of the food canal and their minute spaces (Fig. 5 & 6).

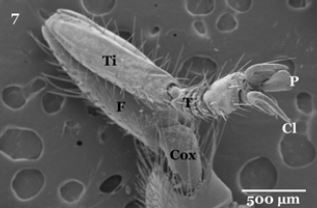
Regarding to their legs, each oneisdivided into tube- like segments, composed of a coxa, trochanter, femur, tibia, and five tarsomeres (tarsal segments) (Fig. 7). The tarsomeres bear many stout sharply setae, along the lateral edges near joints and between segments (Fig. 8). The distal end of the fifth tarsomere bears a pair of curved claws, between the claws of each leg found pad-like structure called pulvilli (PU) and long bristle known as empodium (Fig. 9). Each claw is characterized by sharply strong apexhavingoblique striations on their inner surface (Fig. 10).

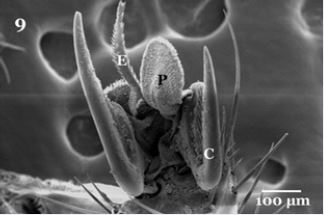

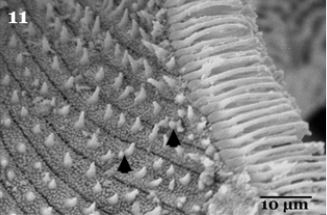
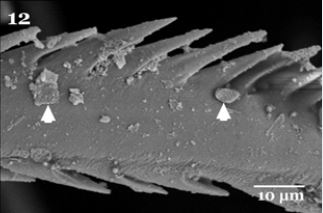
Higher magnification is showing that the ventral surface of pulvilli illustrated heavily packed tenent setae (Fig. 11), characterized by presence of some pointed tip tubercles scattered between the oblique rows of minute tenent setae. The peripheral edges of pulvilli pad is surrounded with several rows of the spoonlike tip tenent setae (Fig.11). The empodium which appears as a wipe -like process (Fig. 9), on higher magnificationis showingsharply pointed spins on both lateral sides (Fig. 12). Also the higher magnification of the pulvilli and empodium cleared presence of minute particles adhering to their ventral surfaces (Fig. 11&12 arrows).
Discussion
Insects spread diseases primarily via stings, bites, infestation of tissues and indirect transmission of pathogens.
Vectors spread diseases by literally carrying pathogens on the surface of their bodies from place to place
[11]. These pathogens transmission in case of intact skin requires contaminated strong piercing parts. Most
piercing proboscides of blood sucking flies have long and thin piercing stylet-shaped structures that are
strongly sclerotized and often apically finely serrate [12] to be capable to pierce vertebrate’s skin and extract
their blood from deeper lying tissues.
As shown in Fig.(5) H. eqiuna has thin long tubular sucking organ armed with prestomal-teeth at the tip that used to pierce the hosts’ skin. These piercing organs are rotated slightly to drill into the tissue. So, it torn open these tissues causing bleeding under skin to be the fly’s feeding [13]. This process of feeding enables the fly for sucking host blood from several to over ten times per a day [14], may reach to 1.5 to 4.5mg with a feeding duration of 3 to 13 minutes [15]. It is suggested that this habitat keeps the labellum moist environments suitable for pathogen survival and proliferation enabling its transmission.
Regarding to Fig. (11), the spoon-like tip of the tenent setae of the pulvilli with sharply pointed spins on the lateral sides of the empodiumand the presence of heavily packed tenent setae of the ventral surface of the pulvilli enabled flies to increase the number of contact points for surface attachment that increasing the probability of microorganism adhesion. Moreover, these adhesive organs have flexible cuticle structures which are able to maximize their effective area of contact on different substrates [16]. The spoon-like tip of the tips of the tenent setae of H. equine adult flies was observed in other fly species such as Chrysomyavilleneuvi [17]. The characteristic of sharp apical claws are used for clinging to softer substrates [18], in addition to some insects have structures on their legs coated with a sticky substance facilitating the insects adherence on non-horizontal surfaces and enhances the adhesion of particles such as bacteria and other microorganisms [19]. They added also Small particles may also readily adhere to an insect’s exterior surfaces due to their electrostatic charges.
The presence of minute particles on the ventral surface of adhesive device (arrows in Fig. 5 & 6) check the possibility of mechanical transmission of pathogens with H. equina. Mechanical pathogen transmission by blood sucking flies depends on the volume of blood left on the mouth parts after feeding [20], and pathogen dissemination by direct contact with contaminated legs, mouthparts, the excreta or regurgitated fluid within a short time after exposure to pathogens [21], moreover the pathogens may be injected with blood sucking arthropod (insects, flies, mites or ticks) saliva after multiplication [12].
Conclusion
In conclusion, the present scanning study of mouth parts and adhesive devices added more information and
clarify the role of H. equinaas a mechanical carrier of many microorganisms and suggesting that H. equina is
not simple mechanical vector, but with detailed complex components.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!