Biography
Interests
Khalid S. Aljabri, MD, FRCPC, FACP* & Emmad I. Alghannami, MD
Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
*Correspondence to: Dr. Khalid S. Aljabri, Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia.
Copyright © 2019 Dr. Khalid S. Aljabri, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The association between type 2 diabetes mellitus (T2DM) with primary (PH) and subclinical
hypothyroidism (SCH) were reported. Thus, we conducted a cross section study to find out the
relationship between T2DM with PH and SCH in Saudi patients with T2DM.
A cross-sectional study was conducted in the Diabetes centre at King Fahad Armed Forces Hospital,
Jeddah, Saudi Arabia from January 2018 to December 2018. Thyroid stimulating hormone (TSH),
free thyroxin (FT4) and HbA1c were measured.
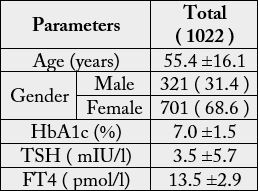
A total of 1022 subjects with T2DM were included in this study. Average age of the study population
was 55.4 ±16.1 years. We found 31.4% were male and 68.6% were female. Mean HbA1c (%),
TSH and FT4 were 7.0 ±1.5, 3.5 ±5.7mIU/l and 13.5 ±2.9pmol/L respectively. PH and SCH
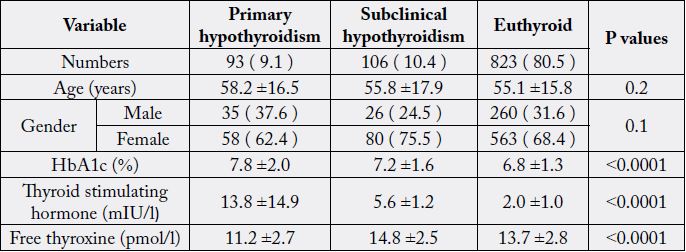
were present in 9.1% and 10.4% where PH was non-statistically significant older than SCH and euthyroid patients (p=0.2). Moreover, females were non-statistically significant more prevalent than
males (p=0.1). HbA1c was statistically significant higher in PH compared to SCH or euthyroid
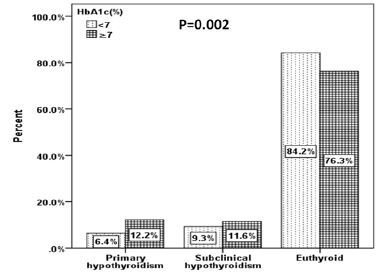
patients (7.8 ±2.0, 7.2 ±1.6 and 6.8 ±1.3 respectively, p<0.0001). HbA1c ≥7% was significant higher
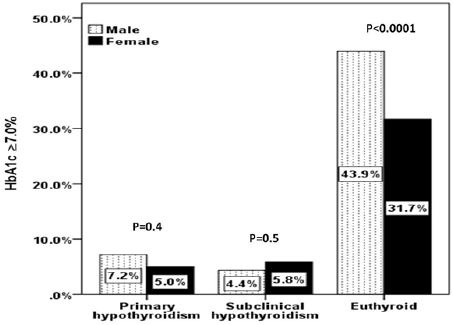
in PH and SCH and lower in euthyroid patients compared to HbA1c <7% (p=0.002). Males were
non-statistically significant higher in patients with PH and HbA1c ≥7% (p=0.4) where as females
were non-statistically significant higher in patients with SCH and HbA1c ≥7% (p=0.5). There was
a trend up of the prevalence of PH and SCH as age advanced where PH was statistically significant
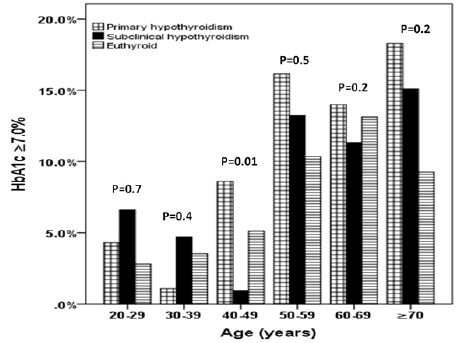
more prevalent than SCH and euthyroid patients (p=0.01) in the fifth decade (figure 3). in the forth
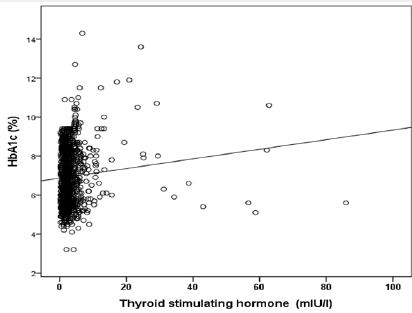
decade (p=0.02). A statistically significant positive correlation was observed between TSH and
HbA1c in the total population (r = 0.097, P=0.002). Also, a non-statistically significant negative
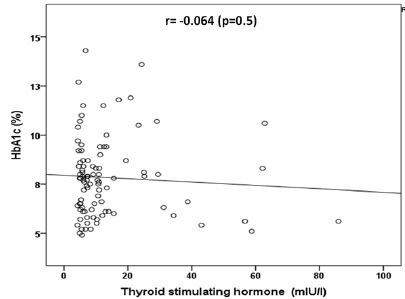
correlation was observed between TSH and HbA1c (%) in patients with primary hypothyroidism
(r = -0.064, P = 0.5). Moreover, a non-statistically significant negative correlation was observed
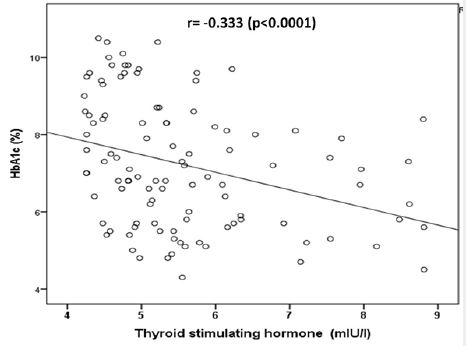
between TSH and HbA1c (%) in patients with subclinical hypothyroidism (r = -0.333, P <0.0001).
PH and SCH are highly prevalent in cohort of Saudis with poorly controlled patients with T2DM.
Introduction
Diabetes and thyroid disorders have been shown to influence each other and associations between both
conditions have long been reported [1-3]. Thyroid disorders are common with variable prevalence. The
prevalence of thyroid dysfunction is higher in diabetics than in controls. In type 2 diabetes mellitus (T2DM),
prevalence of thyroid disease has been found to be as high as 31%, the most common thyroid disorder being
subclinical hypothyroidism (SCH) followed by primary hypothyroidism (PH) [4-9]. In the NHANES
III study, a survey representing the US population, hypothyroidism was found in 4.6% [10]. Perros et al.
demonstrated an overall prevalence of 13.4% of thyroid diseases in diabetics where hypothyroidism was
found in 0.9% [11]. Recently, a prevalence of 12.3% was reported among Greek diabetic patients and 16-
30.7% of Saudi patients with T2DM were found to have thyroid dysfunction [12-14]. In Jordan, a study
reported that thyroid dysfunction was present in 12.5% of patients with T2DM where the prevalence of
hypothyroidism was 6.6% [15].
It is assumed that T2DM is associated with SCH [1,2]. The overall prevalence of SCH is reported to range from 4% to 10% in large general population screening surveys, although it varies with age, sex, and race [10,16,17]. Previous studies suggested SCH was much more likely in patients with T2DM than general population, and the prevalence was reported to be 2.2% to 31% [7-9,11,18-21]. However, some investigators reported no differences between groups and more studies are needed to clarify the relationship between T2DM and SCH [19]. With regard to controversy, few studies compared the SCH of patients with T2DM to that of general population in Saudi Arabia. Moreover, little is known about the prevalence of SCH according to glycemic control status in diabetic patients.
Several studies have been conducted to find out the prevalence of PH and SCH in patients with T2DM but only few studies have compared the levels of glycemic status with PH and SCH in patients with T2DM. The present study is carried out to find out the inter relation between PH and SCH and glycemic status in patients with T2DM in a cohort of Saudi population.
Methods
A cross-sectional study was conducted in the Diabetes centre at King Fahad Armed Forces Hospital,
Jeddah, Saudi Arabia from January 2018 to December 2018 for a period of 12 months which included
1022 patients who were diagnosed as T2DM on the basis of ADA criteria [22]. Patients who are pregnant
were excluded. Thyroid stimulating hormone (TSH) was measured with a chemiluminescent immunoassay
method (CMIA) (Architect i2000 system, Abbott, USA). Serum free thyroxine (FT4) was estimated by
radioimmunoassay. The assays have intra- assay precision of 4.3%. TSH levels between 0.22-4.2mIU/L
and Free T4 12.0-22.0pmol/L were regarded normal [23]. High performance liquid chromatography was
used. HbA1c was expressed as percentage. PH was defined as an elevated TSH >4.2mIU/l and decreased
serum levels of FT4 below the reference range [24]. SCH was defined as an elevated TSH >4.2mIU/l with
a normal level of serum FT4 [24]. The total number of cohort were separated on basis of age values into six
groups: 20-29 years, 30-39, years 40-49, years, 50-59 years, 60-69 years and ≥70 years.
Statistical Analysis
Data are presented as means ± standard deviation (SD) or numbers (%). Quantitative variables were compared
between two groups by using the Student’s test. Differences in categorical variables were analyzed using the
chi-square test. Differences in mean serum 25-OHD levels were tested with ANOVA. The relationship
between continuous variables was assessed using coefficients of correlation. The statistical analysis was
conducted with SPSS version 23.0 for Windows.
Results
A total of 1022 subjects with T2DM were included in this study. Average age of the study population was
55.4 ±16.1 years (table 1). We found 31.4% were male and 68.6% were female. Mean HbA1c (%), TSH and
FT4 were 7.0 ±1.5, 3.5 ±5.7mIU/l and 13.5 ±2.9pmol/L respectively. PH and SCH were present in 9.1%
and 10.4% respectively where PH was non-statistically significant older than SCH and euthyroid patients
(p=0.2) (table 2). Moreover, females were non-statistically significant more prevalent than males (p=0.1).
HbA1c was statistically significant higher in PH compared to SCH or euthyroid patients (7.8 ±2.0, 7.2 ±1.6
and 6.8 ±1.3 respectively, p<0.0001).
HbA1c ≥7% compared to HbA1c <7% was significant higher in PH and SCH and lower in euthyroid patients (p=0.002) (figure 1). Males were non-statistically significant higher in patients with PH and HbA1c ≥7% (p=0.4) where as females were non-statistically significant higher in patients with SCH and HbA1c ≥7% (p=0.5) (figure 2). There was a trend up of the prevalence of PH and SCH as age advanced where PH was statistically significant more prevalent than SCH and euthyroid patients (p=0.01) in the fifth decade (figure 3). in the forth decade (p=0.02) (figure 3).
A statistically significant positive correlation was observed between TSH and HbA1c in the total population (r = 0.097, P=0.002) (figure 4). Also, a non-statistically significant negative correlation was observed between TSH and HbA1c (%) in patients with primary hypothyroidism (r = -0.064, P = 0.5) (figure 5). Moreover, a non-statistically significant negative correlation was observed between TSH and HbA1c (%) in patients with subclinical hypothyroidism (r = -0.333, P <0.0001) (figure 6).
Discussion
Among the endocrinal metabolic diseases, diabetes occupies the major share. There is a complex interaction
between thyroid dysfunction and diabetes mellitus [1-3,5,7,15,18,25]. We found PH and SCH were present
in 9.1% and 10.4% respectively among the 1022 subjects with T2DM. Poor glycemic control was associated
with more prevalent of PH and SCH. In our study we found that diabetic patients have statistically significant
higher prevalence of PH and SCH with uncontrolled diabetes HbA1c ≥ 7 when compared to patients with
HbA1c < 7 (12.1 vs. 6.4%) and (11.6 vs. 9.3%) respectively, p=0.002. These findings are supported by various
studies [26-28]. Due to decreased erythropoiesis, hypothyroidism falsely raises HbA1c [25]. The presence
of both raised levels of thyroid hormones in diabetic may be due to modified thyrotropin releasing hormone
(TRH) synthesis and release [25]. The hyperglycaemia seen in type- 2 diabetics is known to have negative
effect on thyroid function precisely blunting the pituitary TSH response to stimulation by hypothalamic
TRH. This may be due to possible alteration of post translational glycosylation of TRH hence affecting its
biological activity [29]. T2DM is associated with increased insulin level and C-peptide level. Insulin is an
anabolic hormone known to enhance TSH turnover, which is protein in nature [30,31].
Our results showed a non-statistically significant different in both males and females between PH and SCH in diabetics with HbA1c ≥7 as compared to <7. Still, sex hormones can regulate the thyroid function [32]. The difference in sex hormones may partly explain the sex-difference in the relationship between thyroid hormone levels. However, because levels of sex hormones such as testosterone and estrogen were not measured in this study, further research is needed to explore this issue. In addition, because the sample size was smaller for males (31.7%) than in females (68.3%), the precision and statistical power of the analysis may be lower for males.
Our result of positive correlation between HbA1c and TSH is consistent with the results by uppal et al and Velija et al. 33,34 Uppal et al correlated the levels of insulin and HbA1c with thyroid hormones and reported that the levels of HbA1c have a positive and significant correlation with TSH level.33 In addition, we found a negative correlation between TSH and HbA1c in patients with SCH. These findings are supported by various studies who stated that dearrangement in glycemic control influences the thyroid hormone levels [25-27].
We aimed to identify the relation of glycemic control with PH and SCH in Saudi patients with T2DM in hospital-based health care setting. In our study, the observed population reflects a selected yet comprehensive group of patients rather than the general population. In addition, the current study population may appear limited in size and therefore may underestimate the true relation of glycemic control and PH and SCH in patients with T2DM.
Conclusion
We conclude that despite the limitations of this hospital-based retrospective study, PH and SCH are highly
prevalent in cohort of Saudis with poorly controlled patients with T2DM. In the absence of registry data,
larger cooperative studies involving diverse population samples from multiple centers could help to provide
further information on the true relation nationally.
Acknowledgement
The author would like to thank all colleagues from the Department of endocrinology for helping in data
collection.
Funds for study: Nil
Conflict of Interest: None
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!