Biography
Interests
Khalid Aljabri, S.1*, Samia Bokhari, A.1 & Turky Alharthy, A.2
1Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
2College of Medicine, Jeddah, Kingdom of Saudi Arabia
*Correspondence to: Dr. Khalid Aljabri, S., Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia.
Copyright © 2019 Dr. Khalid Aljabri, S., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Vitamin D deficiency and insufficiency are common in youth; especially in youth with type 1
diabetes (T1DM) and type 2 diabetes (T2DM). This study was conducted to determine vitamin D
status among Saudi patients with T1DM and T2DM.
A cross-sectional single centre study was conducted in 421 Saudi patients with diabetes between
January to December 2018. The serum concentration of 25-hydroxyvitamin D (25-OHD) and
HbA1c were measured.
There were 286 patients with T1DM, 155 (54.2%) male and 131 (45.8%) female. The mean age was
16.1 ±2.1 years (12-19 years). The mean and median 25(OH)D concentrations were 43.0 ±22.4 and 37.7nmol/l respectively. The prevalence of different vitamin D status were; 71.3% deficient, 19.9%
insufficient and 8.7% sufficient. Moreover, VDD was significantly more prevalent among males
than females (54.2% vs. 45.8% respectively, p=0.01) with male to female ratio 1.2:1.0. In addition,
there was no statistically significant different between HbA1c and different vitamin D status groups.
The mean 25(OH)D was lowest in the age 18 years patients whereas was highest at the age 19 years (33.5 and 51.9nmol/l respectively). Moreover, males had lower mean 25-OHD than females across most of the age groups with vitamin D deficient was more prevalent at the age 17 years (figure2). 25(OH)D concentration was non-significantly negatively correlated with age (r= - 0.049, p=0.4) and non-significantly positively correlated with HbA1c (r=0.066, p=0.4).
The prevalence of vitamin D deficiency in young with T1DM is high.
Introduction
Type 1 Diabetes mellitus (T1DM) is thought to be the consequence of an autoimmune destruction of the
insulin producing beta cell as a result of interactions between different susceptibility genes and environmental
exposure [1]. The incidence rate of T1DM has grown in Saudi Arabia over the last 3 decades [2]. The
increasing incidence of T1DM strongly suggests the importance of environmental factors; the major factors
being pursued include diet and viruses [3]. The prevalence of type 2 diabetes mellitus (T2DM) in Saudi
Arabia is one of the highest reported in the world, reaching up to 30% in a recent study [4].
The main marker of vitamin D status is the metabolite 25-hydroxyvitamin D (25(OH)D), which is synthesized in the liver [5,6]. Current studies confirm that the prevalence of vitamin D deficiency in the general world population is actually as high as 50-95%, even occurring in countries located in geographical areas which receive sunshine year-round [7]. The Middle East and the North African region in general including Saudi Arabia have very high prevalence of hypovitaminosis D even in the normal asymptomatic population [8-10].
Vitamin D deficiency and insufficiency are common in youth; especially in youth with T1DM and T2DM [11-16]. Proposed mechanisms of an increased prevalence of vitamin D i deficiency and insufficiency include genetic inheritance, increased body mass index (BMI), and concurrent albuminuria with enhanced excretion of vitamin D binding protein and less healthy diets in youth with diabetes [17-20]. In addition to bone health, youth with diabetes may have additional benefits from being vitamin D sufficient. Past research has demonstrated that vitamin D insufficiency/deficiency in pediatric diabetes may contribute to insulin resistance, poor glycemic control, and the development of microvascular and macrovascular complications [13,15,21-26].
At present, however, no data are available about differences of 25(OH)D status between T1DM and T2DM patients in Saudi Arabia. We conducted a cross sectional study to define differences of 25(OH)D status in young patients with T1DM and T2DM.
Methods
A cross-sectional single centre study was conducted in 421 patients with diabetes attended the Diabetes Centre
at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia between January 2018 and December 2018.
Eligible patients were older than 12 years. Exclusion criteria were known hepatic or renal disease, metabolic
bone disease, malabsorption, hypercortisolism, malignancy, immobility for more than one-week, pregnancy,
lactation, and medications influencing bone metabolism. The serum level of 25(OH)D was measured by
competitive protein binding assay using kits (Immunodiagnostic, Bensheim, Germany). Plasma levels from
75.1 to 250nmol/l considered sufficient, from 50 to 75nmol/l insufficient and <50nmol/l as deficient [27]
Glycosylated hemoglobin (HbA1c) was measured by the high performance liquid chromatography method
(Bio-Rad Laboratories, Waters, MA, USA). The study was approved by the ethical board of King Fahad
Armed Forces Hospital.
Statistical Analysis
Data are presented as means ± standard deviation (SD) or numbers (%). Quantitative variables were compared
between two groups by using the Student’s test. Differences in categorical variables were analyzed using the
chi-square test. Differences in mean serum 25(OH)D levels were tested with ANOVA. The relationship
between continuous variables was assessed using coefficients of correlation. Logistic regression analysis was
carried out to identify the independent predictors of vitamin D deficiency considering type of diabetes,
age, gender and HbA1c as risk factors and to estimate odds ratio (OR) and 95% confidence interval (95%
CI). P value <0.05 indicates significance. The statistical analysis was conducted with SPSS version 23.0 for
Windows.
Results
There were 421 patients included in the study. Baseline characteristics are shown in table 1. The study
involved 195 (46.3%) with T1DM and 226 (53.7%) with T2DM (table 1). Male patients were more
prevalent than females in patients with T1DM (61.7% vs. 38.3% respectively) whereas female patients were
more prevalent than males in patients with T2DM (63.8% vs. 36.2% respectively), p<0.0001.
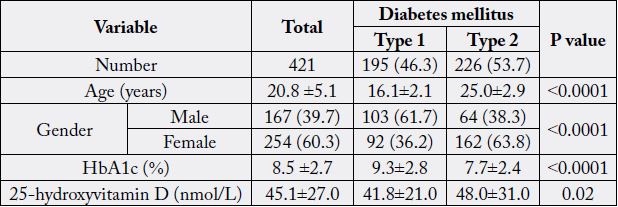
Patients with T1DM have statistically significant higher HbA1c than patients with T2DM (9.3±2.8 vs. 7.7±2.4 respectively, p<0.0001). The mean 25(OH)D concentration was statistically significant lower in patients with T1DM compared to patients with T2DM (41.8±21.0 vs. 48.0±31.0 respectively, p<0.0001).
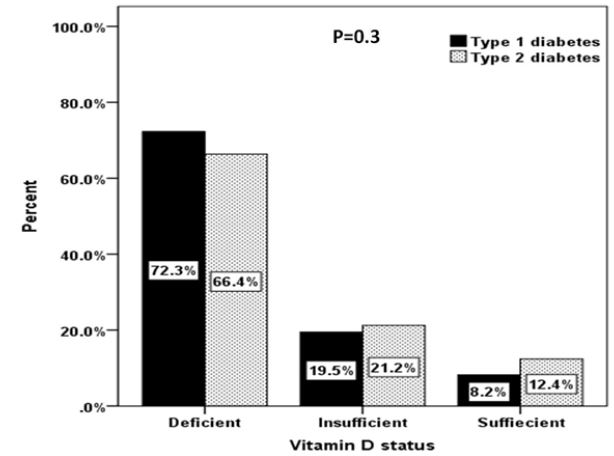
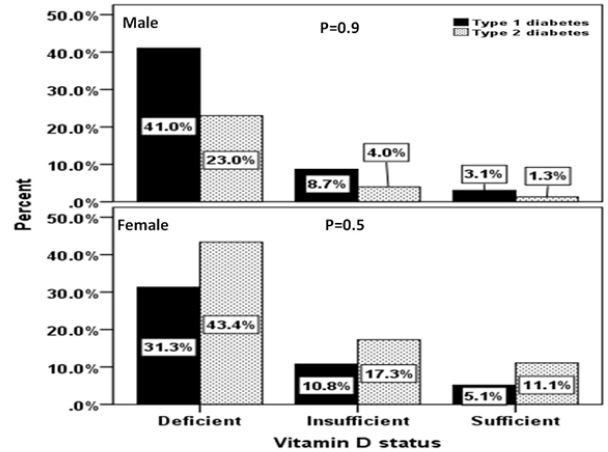
The prevalence of different vitamin D status in patients with T1DM and T2DM were not statistically significant; 72.3% vs. 66.4% deficient, 19.5% vs. 21.2% insufficient and 8.2% vs. 12.4% sufficient (figure 1), p=0.3. Moreover, the prevalence of different vitamin D status in patients with T1DM and T2DM were not statistically significant between both gender (figure 2).
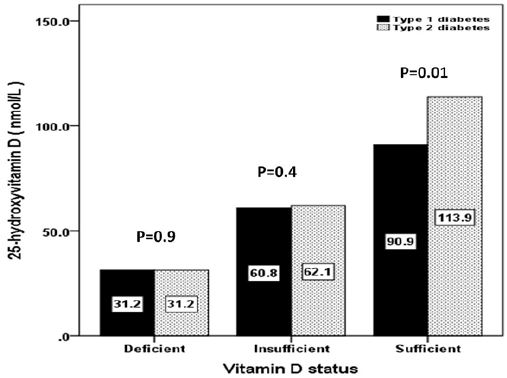
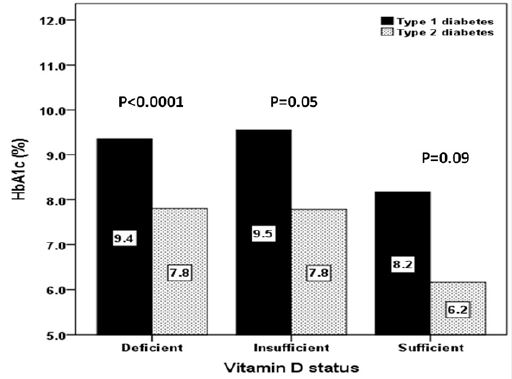
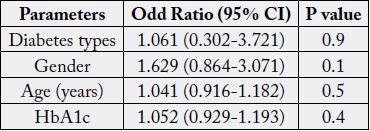
The mean 25(OH)D of different vitamin D status between patients with T1DM and T2DM were not statistically significant in deficient and the insufficient groups but was statistically significant in the sufficient group (figure 3). In addition, the mean HbA1c levels of different vitamin D status between patients with T1DM and T2DM were statistically significant in deficient and the insufficient groups but was not statistically significant in the sufficient group (figure 4). Regression analysis of odd ratio of risk factors for patients with severe vitamin D deficiency showed that type of diabetes, age, gender and HbA1c were not statistically significant associated with vitamin D deficiency (table 2).
Discussion
Diabetes mellitus is characterized by chronic hyperglycemia caused by impaired insulin secretion, peripheral
insulin resistance, or both [28]. T1DM is the consequence of an autoimmune destruction of the insulin
producing beta cell [1]. T2DM is a chronic metabolic disease characterized by the presence of abnormal
blood glucose levels [29]. About 170 million people are affected with T2DM globally and the figures will
increase to 370 million in 2030 [30]. In this cross sectional, single-center study of youth with T1DM and
T2DM, we found that vitamin D deficiency and insufficiency were not differently common (72 vs. 66%
and 20% vs. 21%, respectively). In multivariate analysis, lower vitamin D levels were seen in T1DM youth.
When other variables were taken into account in a multivariate analysis, diabetes type was not significantly
associated with vitamin D deficiency. It is of importance to state that the sample size is representative for a
number of subjects suffering from T1DM and T2DM in the area and study population of one diabetic centre
does not represent the entire city of Jeddah, in addition the study sample confined to patients with T1DM
and T2DM but without comparable groups. Very few foods naturally have or are fortified with vitamin
D, and therefore, the major source of vitamin D for children and adults is sun exposure. With increased
sedentary behaviors occurring indoors and increased use of sunscreen to prevent skin cancer, dietary vitamin
D intake and vitamin D supplements are very important contributors to the vitamin D status of youth [31].
It is a limitation of our study that we did not obtain data on dietary vitamin D intake in our participants
or dose of vitamin D supplements being used by our participants with T1DM or T2DM. Amount of sun
exposure is influenced by season of year and latitude Vitamin D status has been extensively evaluated in
different populations and care settings, with a vitamin D deficiency prevalence of 50-95%, depending on
the used diagnostic criteria and the study population [32-43]. Prevalence of vitamin D deficiency in youth
with T1DM as reported in the literature has varied from 15-68.5%. A study from Boston measured 25(OH)
D levels in 128 children with established and newly diagnosed T1DM. A total of 24% had levels above
75nmol/l, but 61% had levels between 52 to 72nmol/l and 15% were deficient.14 In 110 patients with
T1DM (5-65.1 years of age), 68.5% had a suboptimal vitamin D level ≤ 75nmol/l, and 49% of youth with
T1DM (n=1426) followed in the SEARCH study had deficient vitamin D levels < 50nmol/l.12,13 A study
in Australia by Greer et al. found a three-times higher risk of having levels below 50nmol/l in adolescents
with newly diagnosed diabetes [44]. Pozzilli et al. from Italy examined 25(OH)D levels in 88 children
newly diagnosed with T1DM (mean age 14.6 years) and 57 healthy age and sex-matched controls. The
concentration of 25(OH)D was significantly lower in the diabetic adolescents [45]. Bener et al. compared
25(OH)D levels in 170 age-, race- and sex-matched T1DM cases and healthy controls in Qatar, a region
with ample sunshine all year round. There was a high prevalence of deficiency/insufficiency in both the
groups (90.6 vs 85.3%), but it was significantly higher in the diabetic children [46]. Janner et al, of the 129 children and adolescents with T1DM, 78 (60.5%) were vitamin D deficient, defined as a 25-hydroxyvitamin-
D level below 50nmol/l [47]. A study in Saudi patients with T1DM showed that 71.1% were
severely vitamin D deficient, 20.6% were moderately vitamin D deficient, 8.1% were mildly vitamin D
deficient and 0.2% had normal 25(OH)D levels [48]. Agha et al from Jeddah, Saudi Arabia showed that
48.2% of the diabetic patients were suffering from the condition of VDD [49]. It was also evaluated that
28.8% of the diabetic participants were insufficient. However, 23% of the diabetic participants had normal
levels of 25(OH)D. In contrast, 25(OH)D levels were usually above 50nmol/L in young adults at diagnosis
of T1DM in Sweden [50]. Reports in the literature of the prevalence of vitamin D deficiency in youth
with T2DM are less common. De lasHeras et al. reported that 59% of 27 youth with T2D were vitamin
D deficient with levels < 50nmol/l.15 To our knowledge only Di Cesar et al. compared the prevalence
of vitamin D deficiency < 50nmol/l) in T1DM (n=50) and T2DM (n=63), but the participants were all
adults.16 They found that adults with T2DM had more vitamin D deficiency than adults with T1DM (63.5
vs. 36%). This difference persisted after adjusting for BMI and was unrelated to age, sex, or insulin treatment.
In our cohort, we found that youth with T1DM and T2DM had a comparable prevalence of vitamin D
deficiency and insufficiency. In our study, the three variables, age, gender, and HbA1c, not statistically
significant associated with vitamin D levels. These overall differences might be explained by the variability
of geographical environment, the age of the subjects, duration of diabetes, glycaemic control [14].
We found that no statistically significant difference between vitamin D deficient diabetic patients and those with vitamin D insufficiency or sufficiency regarding age in concordance with other study [51]. It was also noted that the prevalence of vitamin D deficiency increased with age like other reports [51,52]. We found that vitamin D deficiency was statistically significant higher in males than in females in patients with T1DM in concordance with other study [51]. other study found that vitamin D deficiency was more prevalent in females [52]. Male patients may avoid sun exposure as misconception regarding harmful effects of sunlight and unawareness regarding the source of Vitamin D. Earlier study in patients with T2DM in Saudi Arabia showed female patients (73.6%) were vitamin D deficient while (46.9%) of male patients. Other found no significant difference in vitamin D status of males and female [53]. Our result could be attributed to less sun exposure in female patients relative to male patients in our community.
We found that no statistically significant difference between vitamin D deficient diabetic patients and those with vitamin D insufficiency or sufficiency regarding age in concordance with other study [51]. It was also noted that the prevalence of vitamin D deficiency increased with age like other reports [51,52]. We found that vitamin D deficiency was statistically significant higher in males than in females in patients with T1DM in concordance with other study [51]. other study found that vitamin D deficiency was more prevalent in females [52]. Male patients may avoid sun exposure as misconception regarding harmful effects of sunlight and unawareness regarding the source of Vitamin D. Earlier study in patients with T2DM in Saudi Arabia showed female patients (73.6%) were vitamin D deficient while (46.9%) of male patients. Other found no significant difference in vitamin D status of males and female [53]. Our result could be attributed to less sun exposure in female patients relative to male patients in our community.
Our data did not also show any statistically significant difference in HbA1c levels between vitamin D deficient diabetic patients and those with vitamin D insufficiency or sufficient subjects. We are not in line with Sgragg et al. and Hypponen et al. who found that serum 25(OH)D levels were correlated with HbA1c in children with lower vitamin D concentrations [54,55].
We had several limitations. Our study was a cross-sectional study; therefore, it could not evaluate the causal association directly among the studied variables as observed in longitudinal or interventional study. In addition, the study was done at one centre and was done at one point of time. The study sample confined to patients with T1DM and T2DM but without comparable groups. Another limitation in our study includes the limited sample size. The high prevalence of vitamin D deficiency in our study consequently will call for greater sample size.
Conclusion
In conclusion, the prevalence of vitamin D deficiency was not differently high among T1DM and T2DM
subjects. We recommend larger scale studies for detecting vitamin D deficiency in our population with
T1DM and T2DM. From these data, we conclude that the evaluation of vitamin D status should be considered
in patients with T1DM and T2DM. Additional research is needed to confirm whether youth with
diabetes would have benefits, such as improved insulin resistance, glycemic control, and prevention of diabetes
related complication, from vitamin D supplementation [13,15,21-26].
Acknowledgments
There is no any financial support or relationships that may pose conflict of interest.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!