Biography
Interests
Pillay, A. E.
Department of Chemistry, Khalifa University of Science & Technology, Abu Dhabi, UAE
*Correspondence to: Dr. Pillay, A. E., Department of Chemistry, Khalifa University of Science & Technology, Abu Dhabi, UAE.
Copyright © 2019 Dr. Pillay, A. E. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The aim of this communication is to introduce a new opportunity to the medical community in the realm of tumour therapy. The article itself presents a theoretical exposition - into the irradiation of cancerous tumours with charged polyatomic chemical species present in hot plasmas - which could be supported by empirical studies and compared with contemporary charged particle therapy (CPT). The model presented in this communication is a simulated one, based on “molecular irradiation”, and could be of benefit to cancer research.
List of Abbreviations
PTT: Plasma Tumour Therapy
CPT: Charged Particle Therapy
ICP-MS: Inductively Coupled Plasma Mass Spectrometry
ICP-AES: Inductively Coupled Plasma Atomic Emission Spectrometry
RBE: Radiobiological Effectiveness
LET: Linear Energy Transfer
BP: Bragg Peak
Charged particle therapy (CPT) is well known, and well documented [1-4]. Irradiation with plasmas is less known and forms the subject of this article. For more than 30 years plasmas (6000-10000K) have been utilized for analytical purposes [5]. Visualize plasma as the gas on the surface or periphery of the sun. It is an exceedingly hot gas that possesses the capability of atomizing and ionizing all chemical species that come into contact with it. Such atomization could lead to recombination of atomic and ionic entities within the boundaries of the plasma to form completely new heavy charged chemical species [6]. Such species have been detected by mass spectrometers, which imply that they could be accelerated out of the plasma to a specific point. If such a point lies within a cancerous tumour, this could form the basis of plasma tumour therapy (PTT).
Plasmas in ICP-MS (inductively coupled plasma mass spectrometry), and in ICP-AES (inductively coupled plasma atomic emission spectrometry) have been deployed to determine the general elemental composition of various specimens including environmental and biomedical specimens [7]. The plasma is usually hot argon gas, and the existence of a wide variety of polyatomic chemical species in plasmas is outlined in a report by May and Wiedmayer [6]. Some of these charged species or “cluster” ions are presented in Table 1 [6]. These cluster ions exist temporarily in the plasma, and very little is known about their chemistry. How do they bond? What energies are released when they fragment? These are interesting questions that have yet to be answered. Without going into detail, it is known that these species are formed as spectroscopic interferences aspirated into the plasma from components related to the analyte of interest, including the sample matrix, reagents, plasma gases and traces of atmospheric gases. These spectroscopic interferences can be used to advantage. It is necessary to underscore that these interfering polyatomic species occur in “molecular bundles” that do not ordinarily exist in nature. For example: 15N16O1H+, 12C2H+ and 12C4+ are molecular combinations that are impermanently bound, and are expected to fragment into individual particles as it is propelled into a target. If the target is a surface tumour, the cluster could be transported to a depth beneath the surface of the tumour, split, and the fragments impelled in different directions within the tumour. An explosion of particles inside a tumour is a novel concept and the outcome is worth investigating.
The documented literature on oncology and tumour therapy predicates in terms of RBE (radiobiological effectiveness) that carbon ions tend to be more favourable than protons, largely due to the pronounced Bragg Peak (BP) in the case of carbon, which confines linear energy transfer (LET) to a narrowly demarcated region [1-4]. This also entails less lateral damage to normal tissue and tends to be effective for deep-seated targets [3]. Success with heavier ions such as argon is limited [1], and treatment has recently focused on the lighter ions of carbon and oxygen [3,4]. The question arises: what exactly happens when a cluster polyatomic ion is propelled into a tumour? The simulation created to explain this is a fusion-fission model (Figure 1) with its basis in the theory of nuclear fusion and fission [8].
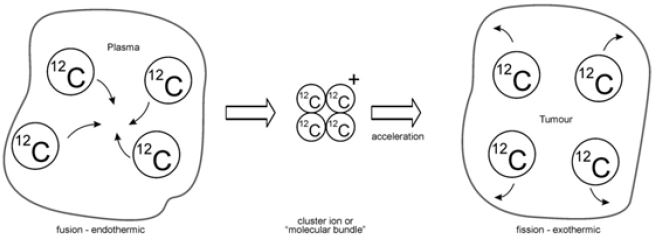
The ideology behind this simulated model is as follows: the atomic entities inside the plasma fuse together endothermically, absorbing energy from the plasma in the process. The process is endothermic for the simple reason that such a combined molecule does not normally exist, is therefore, unstable and energy is derived from the hot plasma to construct it. For purposes of irradiation, the ion is subsequently accelerated, and propelled into the tumour, where is “fissions”, with fragments transported in different directions. The fissioning of the cluster is exothermic, and energy equivalent to its formation and kinetic propulsion is released at the point of fission fragmentation. This could be equivalent to a mini-explosion within the tumour. Let’s consider the case of 12C4+ fragmentation (for example) - energy is disseminated at five points: (i) point of fragmentation, which includes the simultaneous energy released in the break-up of the cluster plus the kinetic energy disseminated at the range of the polyatomic particle; and (ii) at the four BPs of each of the fragmented carbon entities, which could occur at multiple points in the tumour depending on the angles of fragmentation. How is this effective? From the point of killing cells, the RBE is expected to be elevated because energy is simultaneously deposited at multiple points within the tumour. Naturally, the extent of improved RBE has to be explored experimentally. The tracks of the “fission” fragments leave in their wake residual electron scattering, which could somewhat offset the elevated RBE, but controlled by the speed of the target projectile. This would naturally be accompanied by low energy photon emission and x-rays along each track [1,4]. The depth each (carbon) fragmented projectile penetrates within the tumour depends on the total energy released at the point of fragmentation. This would clearly be controlled by the accelerating potential of the cluster ion and the “fission” energy. Bear in mind that the bond energies related to single, double and triple carbon to carbon covalent bonds are roughly 348, 614 and 839kJ/mol, respectively [9]. Nothing exists in the published literature about the nature of the bonding of these cluster ions created in the plasma. It is surmised that clusters ensue from a process of amalgamation resulting from deep fusing of these polyatomic entities, with a “sticking” energy far in excess of bond energies. Directing the projectile into a scintillating solvent or cloud chamber (for example) could produce information on the nature of fragmentation.
Some of the possible merits of plasma tumour therapy (PTT) are summarised below:
1. A distinct advantage is the prospective diminished accelerating potential that could be deployed for greater effect. For example, the effect of 8 MV 12C+ ions could be equivalent to that of 2 MV 12C4 + (or less)? The reduced impact of the projectile could prove to be less noxious to the patient undergoing the treatment. The depth penetration released in the fragmentation process has to be the subject of detailed exploration, but could be greater than that of a singular 12C+ projectile (for example).
2. The interaction may not be classified as entirely nuclear. The cluster ion represents a few atoms held together in a molecular bundle that collapses on impact within the target. The released “fission” fragments are expected to be converted to whole atoms, which are neutral, and which will not interact with nuclei. However, the initial infiltration of the ionic projectile to the substrate of the tumour could lead to short-lived coulomb excitation of light nuclei.
3. One point that could be of considerable benefit to the medical fraternity is that a wide variety of cluster ions can be created within plasmas, for selective use. Table 1 contains but a few of these polyatomic species. The report by May and Widemayer lists more than 450 multiple polyatomic species [6], which could be employed under specific circumstances, such as hypoxic and normoxic situations [1-4]. For example a cluster projectile composed of 16O18O1H+ could be used for penetration of tumours under special circumstances, where each fragment will have its own unique range and RBE based on its individual mass. Other examples of multi-polyatomic species for special types of irradiation include 12C16O2H+, 12C16OH2+, 14N16O18O+, and heavier species such as 29Si2+, 36ArH+, 34S17O18O+. CPT with single charged particles tends to be deficient in certain features. Such deficiencies could be neutralised with PTT using polyatomic charged chemical species.
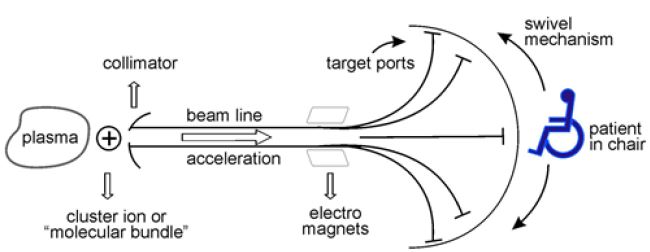
4. Creating these species in the plasma at levels that would be useful for purposes of irradiation would ultimately depend on the skill of the user (especially in generating appreciable molecular ion-beam fluxes). The whole facility comprising the plasma, beam line and other accessories is presented schematically in Figure 2. Several ports for exiting individual polyatomic species could be designed with the patient seated in a carousel that could switch (or swivel) from one port to another.
In the interest of advancing tumour therapy, PTT offers an area of research that could be highly beneficial to humankind. It is an opportunity worthwhile exploring and could make a marked contribution to the medical fraternity.
Declarations
The author thanks Sasi Stephen for his assistance with drawing the figures.
Professor Pillay is skilled in a wide range of instrumental and nuclear techniques including neutron
activation, XRF, gamma ray spectroscopy, ICP-MS, atomic absorption and UV-Vis spectrophotometry. He
is experienced in studies in nuclear and atomic science, and on applications using hybrid plasma mass
spectrometry. He is a Fellow of the Royal Society of Chemistry.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!