Biography
Interests
Stavroula Koulocheri, A.
Department of Biological Chemistry, Medical School, National and Kapodistrian University of Athens, NKUA, Greece
*Correspondence to: Dr. Stavroula Koulocheri, A., Department of Biological Chemistry, Medical School, National and Kapodistrian University of Athens, NKUA, Greece.
Copyright © 2022 Dr. Stavroula Koulocheri, A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This short article describes the recently emerging very weak non-covalent interaction called n → π* interaction and its nature. The brief overview shows the widespread presence of this non covalent interaction in biomacromolecules especially in proteins. Besides the other known non-covalent interactions, n → π* interaction plays a significant role to the thermodynamic stability and functional structure of biological molecules. This knowledge can shed light into the biological processes of living systems with future investigations in other important areas like medicinal chemistry.
Introduction
It is known that noncovalent interactions determine molecular conformation, chemical behavior and
function in biological systems. Electron delocalization plays a significant role in noncovalent interactions. A
novel interaction contributing to protein structure, is called the “n→π* interaction”, deriving from electron
delocalization similar to that of the hydrogen bond. In hydrogen bonding a delocalization of the lone pair
(n) of the hydrogen bond acceptor to the antibonding orbital (σ*) of the hydrogen bond donor takes place
[1,2]. In this noncovalent interaction, called the n→π* interaction, the delocalization referred to the lone- pair (n) electron density of the nucleophile donor group into the empty antibonding orbital (π*) of a nearby
carbonyl group [3-5]. This interaction is similar to the Bürgi-Dunitz trajectory for nucleophilic approach to
carbonyl [6]. The mixing of these orbitals favors attractive interaction releasing energy. The great contribution
to the nature and understanding of the n → π* interaction is due to the works of Raines and co-workers
[7-9].
Nature and Features of n→π* Interactions
n → π* interaction according to the Burgi–Dunitz trajectory of approach of a nucleophile to electrophilic
carbonyl [6] (see Figure), is characterized by O∙∙∙C=O distance (d) of ≤3.2 Å (the sum of van der Waals
radii of carbon and oxygen) and angle ∠O∙∙∙C=O of 109 ± 10°. In the n → π* interaction which is found in
the backbones of proteins [3-5,10-13], the lone pair of electrons on the oxygen atom of a carbonyl group is
delocalized into the π* orbital of a neighbouring carbonyl group [5,14]. n→π* interactions occur in allowed
regions of Ramachandran space and the stereochemical criteria necessary for these n→π* interactions exist
in secondary structures of proteins [5].
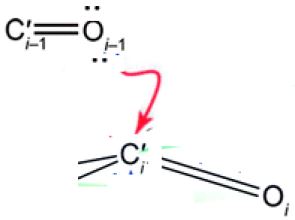
The n→π* interaction creates a partial covalency leading to the pyramidalization of the carbon (C’i) towards the oxygen (Oi–1). Thus, there is a partial covalent bond between Oi–1 and C’i bond in α-helices. The n→π* interaction in α-helices from quantum mechanics [4], induces that pyramidalization of the acceptor carbonyl group and polarization of its π-electron cloud [15].
This finding has important consequences to the folding and conformational stability of α-helices in proteins and this interaction is involved in many protein-ligand interactions [16].
It is well known that a carbonyl group has a planar geometry with the carbonyl carbon and its three attached groups lying in a plane. However, in the n→π* interaction there is some deformation and deviation from planarity towards pyramidization. It is the donor carbonyl that creates this deviation from planarity of the acceptor carbonyl group. The degree of pyramidalization (deviation from planarity) is observed in n→π* interactions in proteins [4,7,11].
This carbonyl deformation introduces a chiral carbonyl structure derived from the otherwise planar, achiral carbonyl group. This chiral signature is unique and very important because this added chiral entity at the backbone amide carbonyl carbon in proteins plays an important role in biomolecular recognition events [17].
The backbone amide carbonyls in an α-helix are involved in an n→π* interaction, inducing lateral compaction of the α- helix.
n→π* interactions are considered relatively weak (∼0.3–0.7 kcal/mol), but the broad presence of carbonyl groups in biology results in a great impact. n→π* interactions are of great importance to protein structure and conformation. Generally one-third of residues in folded proteins have the features to be involved in an n→π* interaction, of particular importance for the α-helix. It is worth of note that in α-helix, there is also a cooperation between hydrogen bonding and n→π* interaction [18].
n → π* Interactions and Their Role in the Backbones of Proteins and the Side Chains
In protein secondary structure, the oxygen (Oi–1) of a main-chain amide is nearby to the carbon (Ci’) of the
successive amide [4,19]. This short contact is enhanced by n→π* electronic delocalization: an oxygen lone
pair (n) overlaps with the Ci’=Oi antibonding orbital (π*) of the subsequent peptide bond [3,20-22].
The n→π* interactions are rich in secondary structures such as α-, 310-, and polyproline II helices. n→π* interactions are found in right- and left-handed α-helices, but not very frequent in β-strands. From peptidic model systems, it has been shown that proline is involved in an n→π* interaction being at the acceptor (i+1) position in both α-helices and ß-strands [3,19].
The amino-acid preferences for n→π* interaction in α-helices follows the order: Pro >Ala > Gly > all other amino acids, with the proline residues more dominant in n→π* interactions. Analogous order for ß-strands in n→π* interactions exists, there is a decrease as Pro >> Gly > all other amino acids [5]. Besides the stabilization of the α-helix, the n→π* interaction should have a great role in α-helix formation as a capable helix initiator [21,23].
Concerning the existence of n → π* interaction in secondary structures of proteins, a computational library of allowed conformations of proteins from works of Raines and co-workers [5], showed their existence. There are many n → π* interactions in the allowed regions of the Ramachandran plot that have the required criteria of distance and angle (≤3.2 Å and 99°-119°, between the carbonyls of residue i-1 and carbonyl of residue i [24]. Generally in α-helices values of C′i-1=Oi-1···C′i distance d ≤ 3.2 Å and angle 100°are prevailed.
The Ramachandran plots created in these studies show that the greater tendency for the n → π* interaction is in left and right handed α-helices. But the existence of the n → π* interaction between the i − 1 and <i residues in β-strands is not significant, however n → π* interaction is observed in twisted β-strands. Thus, the n → π* interaction, in the backbone of about 45% of amino acid residues of proteins, contributes significantly to the stability of the structures of proteins [5].
From bibliography the n → π* interaction, plays a supporting role in the backbone of proteins and folding. It has been recognized from the work of Raines and co-workers the contribution of this interaction to the stability of the collagen triple helix in 2002 [25].
The carbonyl–carbonyl interactions via n→π* interactions, which are found in many protein secondary structures [4,19], cannot be considered as classical electrostatic dipole–dipole [16,26] or charge–charge interactions [27].
Studies in model compounds with cis and trans conformations, show that the trans conformers regarding peptide bond, show closer contact between carbonyl groups and stronger n→π* interaction (there is a greater overlap between the lone pair orbital on the amide oxygen and the empty π* orbital of another adjacent carbonyl group in the trans conformer). Each separate n→π* interaction is small-the range of Ktrans/cis values in solution are of the order of 0.8kcal/mol at 25°C-however, their contribution to protein structure is additive (and could be cooperative) [19].
From the Natural Bond Orbital (NBO) analysis of model chemical structures, there is a sufficient overlap between the electron lone pair orbital on the amide oxygen and the empty π* orbital of another next carbonyl group in a trans conformation.
The value of Ktrans/cis conformation in solutions is referred to the strength of the Ci–1=Xi–1···Ci=Oi interaction. An increase in Ktrans/cis should suggest n→π* electronic delocalization. The degree of pyramidalization corresponds to the strength of the n→π* interaction-and the values of Ktrans/cis are correlated with the relevant overlap between n and π* orbitals.
Generally n→π* interaction influences the conformational stability and folding of the α-helices. In n→π* interaction, because of the short distance between the adjacent carbonyls, a lateral compaction is exerted that induces effective hydrogen bond by the orientation of the distal hydrogen-bond donor and acceptor groups. As in an α-helix, the donor oxygen participates in a hydrogen bond concomitantly with the n→π* interaction.
Besides the contribution to the conformational stability, the n→π* interaction could aid to the folding of an α-helix. In addition the n→π* interaction contributes to the nucleation of an α-helix. The nucleation of an α-helix includes the formation of its first turn, which is not easy in terms of entropy and enthalpy. The n→π* interaction, through adjacent carbonyl groups, can counteract these energetic costs [5]. n→π* interactions do not exist in parallel or anti-parallel β-strands but their presence in β-turns contribute to their conformational stability and folding [28,29].
n→π* interactions are found also present in left-handed helices, which often play a significant role in terms of structure and function. Both are important in substrate specificity, cofactor binding, and protein–protein interactions [30]. It is worth of note that the left-handed α-helical conformation of MPK-3 is important for the binding and ensuing negative regulation of mitogen-activated protein kinase (MAPK) [31].
The n → π* interaction is not only important for the stability but also for the protein function. By the work of Raines and co-workers, there are four n → π* interactions between successive residues in the selectivity filter of the K+ channel from Streptomyces lividans [5].
n→π* interactions are present structurally only in the filter region at low K+ concentration [32].
Besides protein backbone, the n → π* interaction also appears in the side chains of proteins. Sankararamakrishnan and co-workers have examined high resolution crystal structures of many proteins and found interactions between the oxygen atom of the backbone carbonyl group with side chain carbonyl groups, as well as aromatic centers. Also they found interactions between the carbonyl groups of the backbone and side chain of the same amino acid residue aspartic acid from high resolution protein crystal structures in the PDB [33]. These intra-residue contacts between the carbonyl groups are found in about 102 Asp residues. Such interaction with an Asp residue is found in the protein epidermolytic toxin A [33].
Synergism between Hydrogen Bonding and n → π* Interactions
The formation of n→π* interactions acts cooperatively, as the polarization of an acceptorC′i+1=Oi+1
group generates Oi+1 as a better donor. This polarization of the π-bond, in α-helices could enhance i→i+4
hydrogen bond through the carbonyl oxygen as a better hydrogen-bond acceptor.
Maybe the n→π* interactions should promote the flow of electrons through proteins [34].
As reported above, from the NBO analysis by Raines and co-workers, in the n → π* interaction, delocalization of the p-rich lone pair of the oxygen atom of the carbonyl group of residue i − 1 to the π* orbital of the carbonyl group of residue i occurs [5]. It is worth of note that, the s lone pair of the same oxygen is delocalized over the σ* antibonding orbital of the N-H bond of residue i + 4 to form a N-H∙∙∙OC hydrogen bond.
This is called kinship of a hydrogen bond and a n → π* interaction in an α-helix. An example is the oxygen atom of the side chain of an asparagine residue involving in hydrogen bonding with a hydrogen bond donor in addition to a n → π* interaction with a carbonyl group of the main chain of the protein [18]. The n → π* interaction releases about 0.5 kcal mol-1 contributing to the stability of the α-helix. Also this interaction decreases the Oi−1∙∙∙Ci=Oi distance, favoring the hydrogen bond formation. In conclusion the n → π* interaction enhances the strength of the hydrogen bond. This synergism is called positive co-operativity [18].
Conclusions and Outlook
The n→π* interaction is a recently intermolecular interaction that enriches biomolecular structure and
function because of its broad distribution [35]. n→π* interactions stabilize α-helices-by making use the
most of carbonyl lone pairs of the protein backbone. Its description and our understanding is extended to
the synergism of hydrogen bond within the backbones of peptides and proteins. It is expected that n→π* interactions should be found in a range of molecules containing a lot of carbonyl groups, such as various
proteins. This knowledge with the aid of available computational methodologies, can be lead to better
experiments and conclusions. Besides their contribution to the stability of proteins, n→π* interaction play
a significant role to folding of proteins and give some suggestions to different research areas for future
investigation.
The present short article provides an overview of the recent progress of this novel n → π* interaction and its implications in protein structure and function as well as a future outlook on protein -ligand interactions given the fact that n→π* electronic delocalization is involved in many protein-ligand interactions. For example substitution of an amide donor with the appropriate chemical molecule, might provide stronger n→π* interaction (greater overlapping) and result in an increase in ligand affinity in the field of medicinal chemistry.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!