Biography
Interests
Kirsten West, Hien Tran & Yoon Hang Kim*
Medical Director of Integrative Medicine, WellMed, San Antonio, TX 78249, Texas, USA
*Correspondence to: Dr. Yoon Hang Kim, Medical Director of Integrative Medicine, WellMed, San Antonio, TX 78249, Texas, USA.
Copyright © 2021 Dr. Yoon Hang Kim, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Pain presents a tremendous burden to many medical providers, patients, and society as a whole. Specifically, chronic pain greatly affects quality of life, financial security, and healthcare spending. It is estimated that more than 20% of the U.S. population suffers from chronic pain. Of these, more than 7% of chronic pain patients experience being limited in daily activities. The standard approach to treating pain - acetaminophen, NSAIDs, opioids, and gabapentinoids - may be effective at treating acute pain but appear to provide inconsistent and often unsatisfactory relief of chronic pain. In addition, increased opioid use has led to the growing opioid epidemic in the U.S. In an effort to improve efficacy in treating chronic pain, medical providers, researchers, and patients have been exploring the potential use of herbs and supplements in the treatment of pain. Multiple metaanalyses have shown promising results, however, the trials available for review are often associated with study design issues such as: small sample sizes, lack of patient compliance, and inconsistencies among comparable trials. Therefore, the conclusion of these studies is mostly inconclusive and call for better designed trials with larger sample size. In this setting, Corydalis yanhusuo is interesting as it has been used safely as part of Chinese medicine for treating pain. Recently, C.yanhusuo has been shown to mitigate pain due to inflammation and neuropathy without displaying tolerance effect. In this review, the rationale for considering C.yanhusuo as a possible treatment for chronic pain is explored.
Introduction
Pain imposes a tremendous burden to many medical providers, patients, and society as a whole. The estimated
economic cost of pain is more than 500 billion dollars per year which includes direct treatment costs and
lost work hours [1]. In addition, quality of life is negatively affected. Several studies of patient suffering
from chronic back pain and neck pain show that they have lower SF-36 scores in both physical and mental
wellbeing [2,3].
In 2019, the estimated prevalence for chronic pain in the U.S. was more than 20%. Furthermore, more than 7% of chronic pain patients suffer from high-impact chronic pain that limits daily activities [4]. The risk factors for chronic pain include female gender, older age, and rural area residency [5-7].
Pain management tends to occur in a stepwise approach with the first line of treatment including the use of over-the-counter medications such as acetaminophen and non-steroidal inflammatory drugs (NSAIDs). Secondary and prescription medication options include NSAIDs (including COX-2 inhibitors), opioid medications, and gabapentinoids. Unfortunately, the efficacy of these tools for treating chronic pain have been found to be inconsistent and inconclusive [8,9]. In addition, the widespread prescription of opioids may have played a role in creating the opioid epidemic in the U.S. [10].
There has been an effort to step away from relying on solely pharmacological agents for treating pain. The ongoing efforts in the VA hospital, through a program called Whole Health Initiative, is a testament to a more integrative and holistic approach to pain management [11]. In 2017, American College of Physicians made a landmark recommendation that treatment of acute or subacute low back pain should first include non-pharmacologic modalities including; superficial heat, massage, acupuncture, or spinal manipulation [12]. In 2016, Cochrane Reviews published an article which sought to analyze published clinical trials which utilized various herbal preparations in the treatment of pain [13].The results showed that some herbal preparations reduced pain more significantly when compared to placebo, without causing adverse side effects. Unfortunately, these trials showed poor completeness of reporting. The authors called for more well-designed trials with larger sample sizes [13].
Herbs included in the Cochrane Review of Herbal Medicine for Low Back Pain [13]:
• Capsicum frutescens (Tabasco pepper)
• Harpagophytum procumbens (devil’s claw)
• Salix alba (white willow bark)
• Symphytum officnales (common comfrey)
• Solidago chilensis (Anise Goldenrod)
• Lavandula angustifolia (lavender)
An effective yet inadequately studied herb, Corydalis yanhusuo (corydalis, yanhusuo, Asian corydialis), has been widely used for treatment of pain in Chinese Medicine [14]. In 2016, Wang et al published an article describing anti-nociceptive properties of the C. yanhusuo extract [14]. Interesting properties were noted by authors. These properties included efficacy for treatment of neuropathic pain and a lack of tolerance effect, which is often seen with other pain medications. Thus far, there exists limited information in English speaking countries, regarding the use of corydalis for treating chronic pain. Therefore, this review article aims to provide a background and to explore a rationale for considering Corydalis yanhusuo in the treatment of chronic pain.
The below categories outline the current pharmacological management of pain:
1. Non-steroidal anti-inflammatory drugs (NSAIDs) including COX-2 selective inhibitors
2. Anti-nociceptive medications including acetaminophen and opioids
3. Gabapentinoids including gabapentin and pregabalin
Current pain management strategies employ utilization of both anti-inflammatory and anti-nociceptive medications. The first line of therapy in the treatment of mild or moderate pain is NSAIDs or acetaminophen. Second line therapy includes the use of more potent medications, such as opiates. Gabapentinoids agents are utilized for treating neuropathic pain.
Despite the wide use of the above-mentioned pharmacological analgesics, these practices are not supported by outcome studies. A Cochrane review on the use of NSAIDs for acute back pain made the following conclusion [15]:
“NSAIDS seemed slightly more effective than placebo for short-term pain reduction (moderate certainty), disability (high certainty), and global improvement (low certainty), but the magnitude of the effects is small and probably not clinically relevant.”
A 2014 Cochrane review sought to assess the use of opioids in the treatment of chronic low-back pain. The authors concluded that there is some evidence, although of very low to moderate quality, in the shortterm efficacy (for both pain and function) of opioids to treat chronic back pain compared to placebo (16). However, it was also concluded that studies comparing opioids to NSAIDs or anti-depressants resulted in no significant difference. Furthermore, there are no randomized controlled trials (RCT) demonstrating effective and safety of long-term opioid therapy in treatment of chronic low back pain [16]. In conclusion, the efficacy of opioids appears comparable to NSAIDs in the treatment of chronic pain.
It is likely that a combination of analgesic agents may offer synergy and decreased adverse effects due the ability to utilize lower medication dose. This approach is commonly utilized in the administration of anesthesia [17]. In 2013, a Cochrane review evaluated the effects of single dose oral ibuprofen and acetaminophen for acute postoperative pain. The results demonstrated the combination of ibuprofen and acetaminophen to provide superior analgesic effects than either drug alone. Additionally, the need for additional analgesia was lower as was the risk of drug associated adverse events [18]. Another article by Derry, Derry, and Moore demonstrated that the combined benefits of ibuprofen and acetaminophen resulted in reduced use of opioids post total hip arthroplasty [19]. In the current climate of opioid overuse, such a simple, cost-effective, and relatively safe approach makes sense and should be explored for its potential utility in managing chronic pain.
In 2017, a Cochrane review on the use of gabapentin to treat neuropathic pain was published [9]. Wiffen et al concluded that Gabapentin did not offer pain relief in over half of those treated. Additionally, the risk of experiencing an adverse event was high. The authors concluded that three to four out of ten participants who took gabapentin experienced a pain reduction of greater than 50%. This was compared to one to two out of ten participants who took placebo who also experienced a pain reduction greater than 50% [9]. In conclusion, the use of gabapentin for successfully treating and managing neuropathic pain when compared to placebo, appears to be minimal.
It is important to note that the use of analgesic pharmacological medications is not without risk and some,
if not most, have been associated with serious adverse events. These are in addition to high drug dependency
profiles. The adverse effects of NSAIDs include gastrointestinal, cardiovascular, and renal complications
[20]. Opioid use can result in respiratory depression, cough suppression, reduced intestinal motility, nausea,
vomiting, and urinary retention [21]. In addition, tolerance and dependence (psychological and physical)
complicate the chronic use of opioid medications. The adverse effects of gabapentin and pregabalin include
sedation, ataxia, and weight gain [22].
Over the last two decades, the use of alternative medicine has seen tremendous growth in the US. In
2004, Barret published an article which documented that four in ten Americans utilize complementary and
alternative medicine [23]. The number of adults in the United States that used herbs or supplements grew
from 50.6 million in 2002 to 55 million in 2007 [24]. In 2020, the market value of nutraceuticals (herbs,
supplements, and vitamins) is estimated to be at about 90.9 billion dollars [25].
Similar to current analgesic pharmacological practices, the utilization of clinical herbalism commonly
employs a combination of herbs to promote synergy and decrease adverse effects in the mitigation of pain
[26].
In 2004, Soeken published an article on herbs for treating arthritis-related pain [27]. The author concluded the use of H. procumbens (devil’s claw), avocado soy unsaponifiables, capsaicin, chondroitin, glucosamine, and S-adenosyl-L-methionine (SAMe) to be effective in the treatment of pain associated with osteoarthritis and the use of gamma linoleinic acid (GLA) to be effective in the treatment of pain associated with rheumatoid arthritis.
A 2007 Cochrane review by Gagnier et al evaluated the effectiveness of herbal medicines for low back pain [28]. It was concluded that H. procumbens (devil’s claw), S. alba (white willow bark) and Capsicum frutescense (chili pepper) appeared to reduce pain more than a placebo.
The effectiveness of herbal anti-inflammatory agents in the treatment of osteoarthritis and chronic low back pain has also been studied. A 2007 publication concluded that evidence was strong for avocado soy unsaponifiable and H. procumbens (devil’s claw); evidence was moderate for Zingiber officinale (ginger)and Rosa canina L. (rose hip) [29].
In 2010, Rosenbaum published an article on anti-inflammatory dietary supplements for treating osteoarthritis and rheumatoid arthritis [30]. Per study results, the authors supported the use of Devil’s claw for osteoarthritis and omega-3 fatty acids in the treatment of rheumatoid arthritis as with use of these agents, pain mitigation was appreciable.
A more recent 2019 systemic review by Crawford et al sought to delineate the best evidence-based recommendations in pain mitigation [31]. Per review, the authors made conditional recommendations for the following supplements and herbs:
• Avocado Soy Unsaponifiable
• Capsaicin
• Curcuma longa (turmeric) (food form)
• Zingiber officinale (ginger) (food form)
• Glucosamine
• Melatonin
• Polyunsaturated Fatty Acids
• Vitamin D
Of note, the authors made recommendations against supplements and herbs found to be effective in aforementioned previous studies. These included H. procumbens (devil’s claw) and S. alba (White willow) [31]. This inconsistency in clinical evidence further complicates evidence based efforts to identify herbs and supplements in pain management.
The following chart outlines where current evidence stands in the use of natural agents in the management of chronic pain.
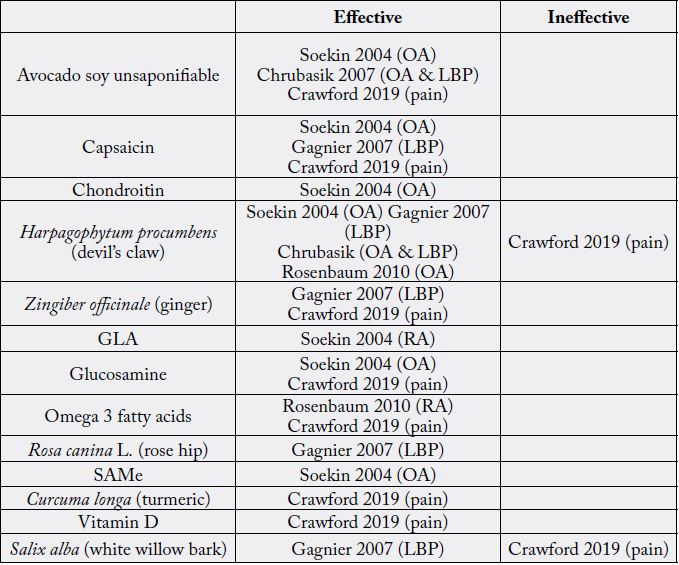
One of the challenges of drawing conclusions on the topic of herbal medicine’s effectiveness for treating pain lies in the lack of high quality, large sample size, randomized, and blinded clinical trials [28]. A 2013 article published by Davidson critiqued the methodology used by Cochrane review in study analysis. Subsequent recommendations were made to account for study methodology and clinical evidence inconsistencies that exist between the use of medications and herbal medicines in pain management [32].
Due to the limited efficacy and poor drug safety profiles of conventional pharmaceuticals for neuropathic
pain, the importance of identifying effective alternative agents is important. Herbal supplements may offer
a viable alternative.
In 2008, Pittler et al published a systematic review evaluating complementary therapies for neuropathic and neuralgic pain [33]. The authors concluded that topically applied capsaicin is effective for relieving neuropathic pain.
Numerous studies have evaluated the use and effectiveness of the Kampo formulation goshajinkan (Chinese pronounciation: niuchesen qi wan) in the treatment of chemotherapy induced neuropathy. However, due to the presence of multiple herbs in the goshajinkan formulation, it proves difficult to identify which herbs offer the most efficacy in mitigating neuropathic pain [34,35]. The goshajinkigan formulation is as follows:
• Rehmanniae radix (Rehmannia root)
• Achyranthis radix (Achyranthes root)
• Corni fructus (Cornus fruit)
• Dioscorearhizome (yam)
• Plantaginis semen (Plantago seed)
• Alisma Rhizome (water plantain)
• Poria Sclerotium (China root)
• Paeonia suffruticosa Andr. (Moutan bark)
• Cinnamomi cortex (Cinnamon bark)
• Powdered processed Aconiti tuber (aconite root)
In 2013 Hao et al published a meta-analysis of ten high quality randomized controlled trials of Chinese Herbal Medicines in the treatment of diabetic peripheral neuropathy [36].The authors reported that due to the clinical heterogeneity and small sample sizes of the reviewed studies, conclusions were elusive. Consequently, the need for well-designed clinical trials remain.
However, even with such approaches, the very nature of Chinese herbal medicines given their many ingredients, make their evaluation in clinical studies arduous. The determination of each herb’s adverse effects versus their combined adverse effects proves difficult. Furthermore, understanding the pharmacological effects of these formulas and single herbs is complicated by inconsistencies of herbal preparation and extraction. However, this evaluation may be necessary to gain leverage as analgesic therapies [26]. These studies may take years if not decades.
C.yanhusuo may offer promise in the treatment of chronic pain. Additionally, its study offers
greater ease than traditional Chinese formulations due to its potency and actions as a single herb.
Corydalis is native to high-altitude grasslands across China. Today it is cultivated. Corydalis was first mentioned in Ben Cao Shi Yi written by Cheng Cang Qi in 720 [37].
It is thought that the primary and active constituent is the alkaloid dehydrocorybulbine (DHCB). DHCB is extracted from the roots of the plant [38]. DHCB has been shown to decrease thermally and chemically induced pain as well as inflammatory and injury-induced neuropathic pain. Its antinociceptive response is similar to that of morphine but without the sedative like effects. However, unlike most pharmaceutical analgesics, DHCB does not generate the tolerance phenomenon [14]. This phenomenon occurs when an increasing amount of medication is required due to physiological medication habituation [14]. Given the tolerance phenomenon as well as sedative like effects of most potent pharmaceutical analgesics, Corydalis is quite remarkable.
Yuan et al published the effects of C.yanhusuo and Angelicaedahuricae (Dahurian angelica) on cold-pressorinduced pain in humans [39].The cold-pressor test is considered a simple, reliable, and widely used model in humans for induction of pain. After a single, oral administration of C.yanhusuo and A.dahuricae, the pain intensity score was shown to significantly decrease (P<0.01). Additionally, the authors noted a doseresponse analgesic effect.
Conclusion
Despite the pharmacological advances in pain management, challenges continue in the treatment of chronic
pain. This is most commonly due to lack of consistent efficacy data with use of standard pharmaceuticals.
Adverse effects and tolerance phenomenon further add to the complexity of pharmacological management
of chronic pain. In this setting, C.yanhusuo possesses promising characteristics that include the ability to
work on both inflammatory and injury-induced neuropathic pain without a tolerance phenomenon. Further
clinical studies are needed to document safety, dose-response, and potential herb-drug interactions.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!