Biography
Interests
Haben Fesseha1* & Tadelech Yilma2
1School of Veterinary Medicine, Wolaita Sodo University, PO box 138, Wolaita Sodo, Ethiopia
2College of Veterinary Medicine, Mekele University, PO box 2084, Mekelle, Ethiopia
*Correspondence to: Dr. Haben Fesseha, School of Veterinary Medicine, Wolaita Sodo University, PO box 138, Wolaita Sodo, Ethiopia. E-mail: tseyon.h@gmail.com, ORCID iD: https://orcid.org/0000-0001-6516-3036
Copyright © 2020 Dr. Haben Fesseha, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Cryosurgery is a type of surgery that involves the use of extreme cold to destroy abnormal tissues. Such type of surgery most often involves the use of liquid nitrogen that has a temperature between -346 and -320°F, although carbon dioxide and argon may also be used. Cryosurgery involves local freezing of tissues for the controlled destruction or removal, by using various action through heat transfer, cell injury, and inflammation. It is typically used for the treatment of equine sarcoids, prostate, angiomas, bovine eye squamous cell carcinoma, oophorectomy in bitches, feline cutaneous squamous cell carcinoma, disbudding in calves. The technique requires minimal post-operative care. Conditions such as skin discomfort, burning sensation of the skin, and hypopigmentation are some of the complications of the technique. Cryosurgery is contraindicated in animals with a history of conditions such as melanoma, compromised circulation, cold intolerance, and cold urticaria. The advantages of cryotherapy are ease of use, low cost, good cosmetic results, and minimal surgical complications. Advancement in cryosurgery technology have dramatically reduced the long-term side effects once associated with the treatment. Yet, more studies are needed on the long-term side effects and effectiveness of cryosurgery.
Introduction
Cryosurgery is a branch of cryobiology and surgery which deals with the therapeutic application of cold
at profoundly low temperatures (those below 0°C) to destroy tissues in selected target sites [1,2]. It is the
use of extreme cold in surgery to destroy abnormal or diseased tissue; thus, it is the surgical application
of cryoablation. The term comes from the Greek words cryo meaning “icy cold” and surgery meaning
“handwork” or “handiwork”. Cryosurgery, also called cryotherapy, is a type of surgery that involves the use of
extreme cold to destroy abnormal tissues, such as tumors. The surgery most often involves the use of liquid
nitrogen, although carbon dioxide and argon may also be used. Cryosurgery has been historically used to
treat several diseases and disorders, especially a variety of benign and malignant skin conditions [3,4].
Cryosurgery is used to destroy problem tissues in the body [4,5]. In most cases of cancer, it’s not the first line of defense. However, it can be used when other forms of treatment have proven unsuccessful, especially if cancer has returned following other treatments such as surgical excision. Cryosurgery is a highly effective treatment for a broad range of benign skin problems. With appropriate instruction and supervised experience, family physicians can master the technique quickly. Cryosurgery is best suited for use in patients with light skin and the treatment of lesions in most non-hair-bearing areas of the body [1,6].
It is, however, used on some internal organs, such as the liver, when disease and other problems make conventional surgery difficult or risky. For instance, it is very useful in the treatment of cutaneous histiocytomas [7]. These benign and often solitary tumors often appear in places where surgical excision can be difficult. It can also be used on chronic inflammatory lesions, such as acral lick granulomas, as an additional treatment modality [8, 9]. It is very effective for treating sebaceous adenoma or nodular sebaceous hyperplasia and one treatment is typically curative with very little follow-up required [6,10,11].
Cryosurgery is used to treat several diseases and disorders, especially skin conditions like warts, moles, skin tags, solar keratoses, and small skin cancers [6,12]. Some internal disorders including liver cancer, prostate cancer, and cervical disorders are also treated with cryosurgery. In the past cryosurgery was more commonly used to treat hemorrhoids. Cryotherapy may be a better description of the procedure since surgery is usually associated with cutting and in most cases, the treatment (therapy) does not involve actual cutting [13].
Cryotherapy is commonly used to treat post-operative inflammation, musculoskeletal trauma, and muscle spasms and to minimize secondary inflammation following therapeutic exercise. It induces a temperature change in the affected tissue of between 1 and 4 intramuscularly and 12-13 at the skin surface as heat is removed from the body. The general advantages of cryotherapy are its ease of use, low cost, and good cosmetic results [4,9,14].
Cryosurgery is used as the primary treatment for early prostate cancer that’s contained in the prostate. It’s also performed when cancer returns after other therapies. Besides, warts, moles, skin tags, solar keratoses, molluscum, [15] Morton’s neuroma [5], and small skin cancers are candidates for cryosurgical treatment. Several internal disorders are also treated with cryosurgery, including liver cancer, prostate cancer, lung cancer, oral cancers, cervical disorders, and, more commonly in the past, hemorrhoids. Soft tissue conditions such as plantar fasciitis [16] (Jogger’s heel) and fibroma (benign excrescence of connective tissue) can be treated with cryosurgery. Although found to be effective, this method of treatment is only appropriate for use against localized disease, and solid tumors larger than 1cm. Tiny, diffuse metastases that often coincide with cancers are usually not affected by cryotherapy [2,17].
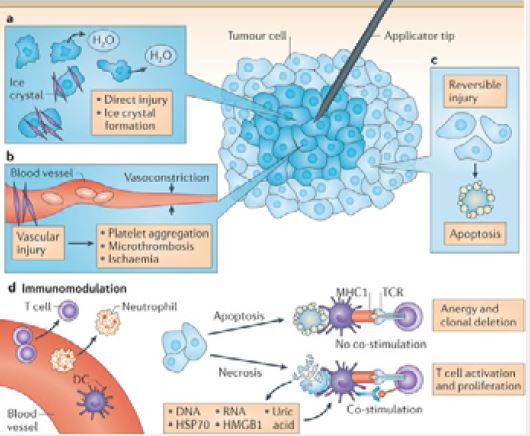
Cryosurgery works by taking advantage of the destructive force of freezing temperatures on cells. When their temperature sinks beyond certain level ice crystals begin forming inside the cells and, because of their lower density, eventually tear apart those cells. Further harm to malignant growth will result once the blood vessels supplying the affected tissue begin to freeze [1,11].
Cryosurgery is also used to treat internal and external tumors as well as tumors in the bone. To cure internal tumors, a hollow instrument called a cryoprobe is used, which is placed in contact with the tumor. Liquid nitrogen or argon gas is passed through the cryoprobe. Ultrasound or MRI is used to guide the cryoprobe and monitor the freezing of the cells. This helps in limiting damage to adjacent healthy tissues. A ball of ice crystals forms around the probe which results in freezing of nearby cells. When it is required to deliver gas to various parts of the tumor, more than one probe is used. After cryosurgery, the frozen tissue is either naturally absorbed by the body in the case of internal tumors, or it dissolves and forms a scab for external tumors [18].
Cryosurgery is a minimally invasive procedure, and is often preferred over the traditional kinds of surgery because of its minimal pain, scarring, and cost; however, as with any medical treatment, there are risks involved, primarily that of damage to nearby healthy tissue. Damage to nerve tissue of the skin is of particular concern. Patients undergoing cryosurgery usually experience redness and minor-to-moderate localized pain, which most of the time can be alleviated sufficiently by oral administration of mild analgesics such as ibuprofen, codeine, or acetaminophen (paracetamol). Blisters may form as a result of cryosurgery, but these usually scab over and peel away within a few days. Occasionally, hypopigmentation may occur in the area of skin treated with cryosurgery, however, this complication is usually transient and often resolves as melanocytes migrate and repigment the area over several months [3,6].
In contrast to conventional surgical methods, cryosurgery provides various advantages such as the quick and technically simple method of tumor removal, the short duration of surgery, bloodless surgery, surgery without scar formation, minimal operative and anesthesia trauma, surgery without a scalpel, early removal of tumor cell before any metastasis develops and prevent the spread at the time of excision of the tumor [9,19]. Thus, this review aims to highlight the principle, technique, application, and complication of cryosurgery.
The controlled destruction of tissue by freezing is today widely practiced in medicine [20]. Terms for it
include cryotherapy, cryocautery, cryocongelation, and cryogenic surgery, but cryosurgery (literally, cold
handiwork) seems most appropriate. Cryosurgery is a cheap, easy, and safe treatment suitable for both
hospital and office-based practice. Its major advantage is excellent cosmetic results with minimal scarring.
The benefits of cold have been appreciated for many thousands of years. The ancient Egyptians, and later
Hippocrates, were aware of the analgesic and anti-inflammatory properties of cold. Over the past 200 years,
cold treatment has evolved from generalized applications such as hydrotherapy to specific, focal destruction
of tissue today’s cryosurgery [2,3].
James Arnott (1797-1883), an English physician, published on the use of cold between 1819 and 1879 [19,21]. He was the senior physician of Brighton Infirmary but moved to London on winning fame. His brother, a scientist, had already gained fame and fortune as the inventor of the slow combustion stove. Arnott was the first person to use extreme cold locally for the destruction of tissue. He used a mixture of salt and crushed ice (`two parts finely pounded ice and one part of chloride of sodium for palliation of tumors, with resultant reduction of pain and local hemorrhage. He stated that a very low temperature will arrest every inflammation which is near enough to the surface to be accessible to its influence [21].
He designed his own equipment, consisting of a waterproof cushion applied to the skin, two long flexible tubes to convey water to and from the affected part, and a reservoir for the ice/water mixture and a sump. He exhibited this at the Great Exhibition of London in 1851 and won a prize medal for his effort [4,19]. (The Great Exhibition was a showcase for the Empire’s scientific prowess not unlike the Millennium Dome but with considerably more style.) Arnott treated breast cancer, uterine cancers, and some skin cancers. Although palliation was his main aim he recognized the potential of cold for curing cancer, stating that the cases he had seen `are, therefore, by no means unfavorable to the supposition of the curability of cancer by congelation’. He advocated cold treatment for acne, neuralgia, and headaches, achieving temperatures of -24°C. Besides, he recognized the analgesic `benumbing’ effect of cold, recommending the use of cold to anesthetize skin before the operation. He was concerned about the safety of the new anesthetic agents that were being introduced and advocated the use of cold as an alternative. This was to become a lifelong crusade that was ultimately unsuccessful, but his contribution to the development of cryosurgery was crucial [11,20].
Cryotherapy, also called cryosurgery, cryoablation, percutaneous cryotherapy, or targeted cryoablation therapy
is a minimally invasive treatment that uses extreme cold to freeze and destroy diseased tissue, including
cancer cells [9]. Although cryotherapy and cryoablation can be used interchangeably, the term “cryosurgery”
is best reserved for cryotherapy performed using an open, surgical approach. During cryotherapy, liquid
nitrogen or high-pressure argon gas flows into a needle-like applicator (a cryoprobe) creating intense cold
that is placed in contact with diseased tissue. Physicians use image-guidance techniques such as ultrasound,
computed tomography (CT), or magnetic resonance (MR) to help guide the cryoprobes to treatment sites
located inside the body [5,11].
Cryotherapy is a controlled and targeted destruction of disease tissue by the application of cold temperature substance. It is used for the treatment of diverse benign lesions and well-circumscribed premalignant and malignant tumors [17]. Cryotherapy uses imaging guidance, a needle-like applicator called a cryoprobe, and liquid nitrogen or argon gas to create intense cold to freeze and destroy diseased tissue, including cancer cells. It may be used to treat a variety of skin conditions as well as tumors within the liver, kidneys, bones, lungs, and breasts [13].
Cryotherapy destroys diseased tissue located outside the body by directly applying the liquid nitrogen with a cotton swab or spray device. For tumors located below the skin surface and deep in the body, the physician will use image-guidance to insert one or more applicators, or cryoprobes, through the skin to the site of the diseased tissue and then deliver the liquid nitrogen or argon gas. Percutaneous image-guided procedures such as cryotherapy are most often performed by a specially trained interventional radiologist in an interventional radiology suite or occasionally in the operating room [1,17].
Principles of Cryobiology
Mammalian cells are destroyed when cooled to a temperature of -20°C (-4°F). Primary injury begins with
the formation of ice crystals, both intracellular and extracellular. The cell’s outer membrane becomes ruptured
by intracellular crystals, and ice formation outside the cell dehydrates the cellular environment, resulting in
lethal electrolyte concentrations and pH changes. When organelles are damaged, the cell loses its ability to
regulate ion permeability and cell death ensues [4,22].
Secondary injury from freezing occurs from vascular stasis. As the permeability of vessels is increased, loss of plasma causes local hemoconcentration. Damaged endothelium in arterioles and venules induces thrombus formation of the vessels, and infarction of frozen tissue occurs within hours of freezing. The cryogenic lesion is a volume of coagulation that closely responds to the extent of the induced ice ball. Rapid freezing results in the greatest intracellular concentration of ice. Thereafter, slow thawing of the tissue results in recrystallization, during which small crystals enlarge, producing more cell damage. To ensure that all targeted tissue receives a lethal dose of cold, a second freeze/thaw cycle is used. Because precooled tissue freezes faster than normal tissue, repeating this cycle causes necrosis of the target tissue more consistently [9,23].
Variations in vascularity, noncellular structure, and water content cause tissues to respond differently to cryonecrosis. Dry tissues (e.g., the cornea) do not readily form ice crystals and therefore do not respond to cryotherapy very well. The cellular components of peripheral nerves are destroyed by freezing, but because the fiber scaffolding of the epineurium is not damaged, regeneration is possible [7,24]. Tissues near major blood vessels or in highly vascular areas are difficult to freeze rapidly and tend to thaw quickly without loss of function [15].
The use of a small amount of epinephrine or temporary regional vessel occlusion may be necessary to ensure proper treatment in those tissues. Future developments in cryotherapy may include the use of nanoparticles to improve freezing efficiency. The basic principle is to deliver these particles into target tissues to maximize the freezing heat-transfer process, to regulate freezing scale, to modify ice-ball formation, to enhance iceball margin ultrasonographic imaging, and thus to prevent healthy tissues from being frozen [5,22].
Immune responses directed against tumor cells have been documented after cryosurgery, and the cryoablationinduced anticancer immune reaction is a well-documented phenomenon in people and other animals [6,19]. Although this has not been proved clinically in horses, numerous case reports suggest secondary tumor regression does occur as a result of the cryosurgical treatment of a primary tumor [1,25].
Although liquid nitrogen, nitrous oxide, and carbon dioxide are all cryogens used in veterinary medicine, liquid nitrogen is the most versatile and therefore the most commonly used. Liquid nitrogen has a boiling point of -195.8°C (-320.4°F). Cryogens, usually stored in liquid form in Dewar flasks can be delivered as a spray or used by super-chilling a probe. Two types of probes are used: hollow probes and solid probes. When hollow probes are used, the liquid is circulated through the probe and exits under pressure through a small opening. When solid probes are used, they are chilled by immersion into the liquid cryogen [3] (Figure 1).
Cryotherapy can be applied topically (on the skin surface), percutaneously, or surgically. Topical cryotherapy
is used typically in the case of skin and eye lesions. When the lesion is situated below the skin surface, a
needle-like therapy probe or applicator needs to be placed through the skin. Occasionally, a surgical incision
may be required. Cryotherapy is used to treat different diseases and abnormalities such as skin tumors, precancerous
skin moles, nodules, skin tags, unsightly freckles, and retinoblastomas [4,23].
Besides, it is also used to treat diseases like prostate, liver, and cervical cancers, especially if surgical resection is not possible. Cryotherapy is also being used to treat tumors in other parts of the body, such as the kidneys, bones (including the spine), lungs, and breasts (including benign breast lumps called fibroadenomas). Although further research is needed to determine its long term effectiveness, cryotherapy has been shown to be effective in selected patients [1,26].
Cryosurgery does not require a sterile field. Therefore, it is a good choice for the treatment of benign and neoplastic cutaneous lesions [27]. By far the most common tumor that is treated with cryotherapy is the equine sarcoid [28]. However, a plethora of skin conditions amenable to surgery can be treated by cryotherapy. Because there is frequently no need for general anesthesia of horses afflicted with skin lesions, cryosurgery has an advantage over other types of surgical extirpations; it frequently can be done on an outpatient basis [17,29].
Cryotherapy, also known as cryosurgery, is a commonly used in-office procedure for the treatment of a
variety of benign and malignant lesions [10,25]. The mechanism of destruction in cryotherapy is necrosis,
which results from the freezing and thawing of cells. Tissue injury is produced by a sequence of destructive
effects, beginning with prolonged tissue cooling, metabolic disruption, ice crystal formation and cellular
rupture. After thawing, microcirculatory failure and the associated ischemia add to cell death, resulting in
a coagulative necrosis. Physical processes of destruction are effective immediately, but physiological-based
detrimental effects, including cytokine release and induction of apoptosis and secondary necrosis, produce a
damaging effect over several days [23,30].
The basic principles of cryosurgery for tumors are fast cooling of the tissue to a cell-lethal temperature, slow thawing, and repetition of the freeze-thaw cycle. Ideally a temperature of -40 °C should be produced at the tumor margin to ensure that all portions of the tumor are subjected to lethal conditions. Repetition of freezingthaw cycle elevates the cell-lethal temperature due to additive cellular stress, as does an increased duration of freezing [30].
The adverse effects of cryotherapy are usually minor and short-lived. Dermatologists have used cryotherapy since the turn of the century. After the development of the vacuum flask to store subzero liquid elements, such as nitrogen, oxygen, and hydrogen, the use of cryotherapy dramatically increased. The mechanism of action in cryotherapy can be divided into 3 phases: (1) heat transfer, (2) cell injury, and (3) inflammation [2,9,11] (Figure 1).
The mechanism by which cryotherapy destroys the targeted cells is the quick transfer of heat from the
skin to a heat sink. The most commonly used cryogen is liquid nitrogen, which has a boiling point of
-196°C. The rate of heat transfer is dependent on the temperature difference between the skin and the liquid
nitrogen. When using the spray cryotherapy technique, the liquid nitrogen is applied directly on the skin,
and evaporation (boiling heat transfer) occurs in which the heat in the skin is quickly transferred to the
liquid nitrogen. This process results in the liquid nitrogen evaporating (boiling) almost immediately. When
using a cryoprobe for cryotherapy, conduction heat transfer occurs where the heat is transferred via the
copper-metal probe [9].
Cell injury occurs during the thaw after the cell is frozen. Because of the hyperosmotic intracellular
conditions, ice crystals do not form until -5°C to -10°C. The transformation of water to ice concentrates the
extracellular solutes and results in an osmotic gr,adient across the cell membrane, causing further damage.
Rapid freezing and slow thaw maximize tissue damage to epithelial cells and are most suitable for the
treatment of malignancies. Fibroblasts produce less collagen after a rapid thaw [31]. Therefore, a rapid thaw
may be more suitable for the treatment of keloids or benign lesions in areas prone to scarring [32].
Freeze damage can be seen when a steak is defrosted from the freezer. The steak juices that are seen when fully thawed represent the intracellular liquid that has escaped because of the damage to the cell wall. Low temperature also ensures maximum damage by further concentrating electrolytes intracellularly. Keratinocytes need to be frozen to -50°C for optimum destruction. Melanocytes are more delicate and only require a temperature of -5°C for destruction. This fact is the reason for the resulting hypopigmentation following cryotherapy on darker-skinned individuals. Malignant skin cancers usually need a temperature of -50°C, while benign lesions only require a temperature of -20°C to -25°C [9].
The last response to cryotherapy is inflammation, which is usually observed as erythema and edema [23].
Inflammation is the response to cell death and helps in local cell destruction. A thorough cryotherapy
treatment causes basement membrane separation, which may result in blister formation [1,9].
Self-pressurizing spray guns (Figure-2) deliver a combination of vapor and droplets of liquid cryogen and
are the most effective method of cryogen delivery. As liquid nitrogen contacts the tissue, it evaporates or
changes from the liquid to the gas phase. This has been shown to remove a greater amount of heat from
treated tissue than is achieved with probes. The volume and size of the spray droplet are controlled by the
diameter of the needle orifice and the trigger in the pressurizing gun. The spray cryotherapy technique is
probably the most commonly used method. This method is suitable for most benign and some superficial
neoplastic lesions. Pulsing each spray to avoid overexpansion of the treatment site prevents complications
[9].

The surgeon can gauge the volume of the cryogen so that the wetting conforms to the shape of the tumor’s surface. However, care must be taken to prevent excess liquid cryogen from running off onto the surrounding skin. It is common to pack the surrounding area with Vaseline-impregnated sponges to prevent this runoff. Alternatively, a spray cup can be used that has the advantage of controlling runoff. Cup size is chosen that fits over the tumor, and as the spray is applied, droplets form a liquid pool over the tumor [6].
A thin film of white petrolatum can be applied to the treatment site, and the cold probe is firmly pressed
against the lesion. Many practitioners do not use the probe because it is cumbersome and time-consuming.
It is reported that good clinical outcomes are observed with probe-delivered cryotherapy in patients with
primary malignant bone tumors [27]. Manufacturers have devised various metal attachments to serve as
heat-conducting probes for cryotherapy. Copper, because of its high conductivity, is typically used [9].
Hollow probes are cooled by circulating a liquid cryogen through them. Hollow probe freezing is easiest to control, but the rate at which it cools an area is slow compared with the rate achieved by spray and solid probes. Hollow probes can be used for either contact or penetration freezing, depending on the configuration of the probe. During freezing, traction can be used to lift the tumor away from underlying structures as an ice ball is extended to the monitored limits. Cryoprobes (figure-3) frequently are used in the treatment of smaller facial lesions (e.g., on the eyelids), where scatter of liquid nitrogen is undesirable. Probes also are useful in the management of vascular lesions, where the pressure of the probe can be used to remove blood from the tissues thus allowing more adequate treatment [6].
Techniques of Cryosurgery
Preoperative evaluation of the patient should be implemented before cryosurgery. This is step is imperative
and necessary standards of care, appropriate cytological, and histopathological diagnostic steps should be
performed according to the indicated procedure. If a mass is deemed malignant with metastatic potential,
addressing the mass with aggressive surgical intervention, radiation and chemotherapy would be indicated
based on oncologist recommendations should the patient’s owner elect to pursue that line of treatment.
Once a mass has been diagnosed, the use of cryosurgery can be employed for nearly any lesion on the skin,
as well as some mucus membrane tissues. Prior to freezing the lesion, the patient must be informed of the
procedure, and verbal or written consent must be obtained. Most of the time, cryosurgery is done without
sedation or general anesthesia, the locations of some lesions will still require chemical immobilization to
achieve desired results. Besides, a local anesthetic, such as 1% lidocaine, or a topical anesthetic can be used
[17].
When using either contact or penetration cryotherapy, monitoring the depth of freezing can be done either by subjective inspection or by objective measurement of temperature changes. Subjective assessment is made by visual inspection or palpation of the ice ball. The outer edge of the ice ball is about 0°C (32°F), which is inadequate for tissue destruction. Seventy-five percent of the tissue within an ice ball is destroyed by freezing.
The depth of contact freezing is estimated to be slightly less than the radius of the ice ball. Pyrometers can be used to measure the temperature achieved beyond the limits of the target tissue. Single- or multiple-channel monitors are available. Needle probes are placed into the tissue adjacent to the deepest portion of the target. When temperatures of -20°C (-4°F) are recorded, all unwanted tissue is destroyed. Alternatively, ultrasonic evaluation of the margin of the ice ball can be an accurate method of determining the extent of the freeze. If the tumor has a distinct ultrasonic appearance, it enables more accuracy in controlled freezing [3,33].
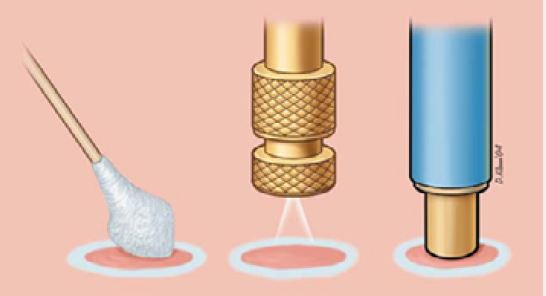
Various treatment modalities have been devised in the use of cryotherapy of lesions. They include the spray freeze technique, the applicator technique, the cryoprobe method, and the thermo-coupler method. (figure 4). Liquid nitrogen is the best and universal freezing source because of its low boiling point and its ease of use. Other sources that are used to freeze, such as Freon, carbon dioxide, and nitrous oxide, do exist, but they are not as efficient in destroying lesions because of their higher boiling points; details of these are illustrated as follows [3,5,34].
Liquid Nitrogen
A common method of freezing lesions is using liquid nitrogen as the cooling solution. This -196°C (-321°F)
cold liquid may be sprayed on the diseased tissue, circulated through a tube called a cryoprobe, or simply
dabbed on with a cotton or foam swab. Cryotherapy uses nitrogen or argon gas to create extremely cold
temperatures to destroy diseased tissue. To destroy diseased tissue located outside the body, liquid nitrogen
is applied directly with a cotton swab or spray device. For tumors located below the skin surface and deep
in the body, the physician will use image-guidance to insert one or more applicators, or cryoprobes, through
the skin to the site of the diseased tissue and then deliver the liquid nitrogen or argon gas [11,28].
The dipstick applicator method is the original method used to apply liquid nitrogen to lesions. A cottontipped
applicator is dipped into liquid nitrogen from a polystyrene cup. The dipstick applicator is then
firmly pressed against the lesion for the desired duration. Low temperatures are not achieved in the dipstick
applicator method as they are in the spray technique; therefore, this method is suitable only for benign
lesions. Adenovirus is capable of survival in liquid nitrogen; therefore, the same source of liquid nitrogen should not be used with different patients. Previously unused liquid nitrogen can be placed in a polystyrene
cup and then promptly discarded after use for a single patient [35].
To treat malignant lesions, a temperature probe coupled to a digital thermometer that can read to -75°C can
be used. A local anesthetic is injected into the lesion, and a temperature probe is inserted into the estimated
depth of the lesion. Usually, a metal or styrene cone is used to concentrate the freeze. The liquid nitrogen is
sprayed into the cone until the desired temperature is reached, usually -50°C to -60°C. This process can be
repeated until the desired destruction is achieved [9].
Carbon dioxide is also available as a spray and is used to treat a variety of benign spots. Less frequently,
doctors use carbon dioxide “snow” formed into a cylinder or mixed with acetone to form a slush that is
applied directly to the treated tissue [4,12,19].
Recent advances in technology have allowed for the use of argon gas to drive ice formation using a principle
known as the Joule-Thomson effect. This gives physicians excellent control of the ice and minimizes
complications using ultra-thin 17 gauge cryo needles [10].
A mixture of dimethyl ether and propane is used in some “freeze spray” preparations such as Dr. Scholl’s
Freeze Away. The mixture is stored in an aerosol spray-type container at room temperature and drops to
-41°C (-42°F) when dispensed. The mixture is often dispensed into a straw with a cotton-tipped swab.
Similar products may use tetra fluoro-ethane or other substances [9].
The spray cryotherapy technique is probably the most commonly used method [6]. This method is suitable for most benign and some superficial neoplastic lesions. Pulsing each spray to avoid overexpansion of the treatment site prevents complications. The cone attachments are round; therefore, they are most suitable for round lesions. For large, irregularly shaped lesions, sequential freezing is necessary [3,17].
Forceps or clamps techniques can be used to concentrate the freeze and to prevent collateral damage. A
clamp is being used during the freezing treatment. Usually, 2 freeze applications of 15 seconds are required
[36].
Benefits of Cryotherapy
When an open surgical approach is taken, the recovery time following cryosurgery of kidney or liver tumors
may be less than for open, surgical removal of the tumor. For percutaneous cryotherapy, the patient may stay
overnight or be released several hours after the procedure. Overnight stays for pain control are usually not
needed. Percutaneous cryotherapy is less traumatic than open surgery since only a small incision is needed
to pass the probe through the skin, which limits damage to healthy tissue. Consequently, percutaneous
cryotherapy is less costly and results in fewer side effects than open surgery. A patient usually can resume
activities of daily living 24 hours after the procedure, if not sooner. However, caution about heavy lifting may
extend for several days after abdominal treatment. For the treatment of fibroadenomas, cryotherapy causes
minimal scar tissue and no apparent post-treatment calcifications [13,37,38].
In vitro and in vivo experiments on the molecular basis of cell death associated with cryosurgery have demonstrated the potential value of adjunctive cytotoxic chemotherapy. The idea is to increase the extent of injury to cells in the peripheral portion of the cryogenic lesion with the expectation that differences in cell sensitivity to freezing may be mitigated. The objective of these strategies is to “make ice lethal at 0°C”. This would provide for an ablative event throughout the entire target region, markedly reducing the potential of disease reoccurrence from satellite populations of cancer cells surviving within the targeted frozen region. Further research should lead to a better understanding of the molecular mechanisms involved in cryosurgery and adjunctive therapy, which in turn should increase the efficacy of cryosurgery for tumors [23,30,39].
Complications and Risk of Cryosurgery
Cryotherapy is an alternative cancer treatment when surgical removal of a tumor may be difficult or, for some
patients, impossible. But its long-term effectiveness is still being examined. Currently, little published data
deal with the long-term results of percutaneous cryotherapy but long-term follow-up for prostate cancer
suggests cancer-control rates are similar to surgery or radiation therapy [9]. Cryotherapy is considered a
localized therapy. It can only treat disease at a single site. It cannot treat cancer that has spread to other parts
of the body [19,40]. Because physicians treat the tumors they see on radiologic images, microscopic cancer
may be missed. Although its use in the bone, kidney, liver, and lung is promising, percutaneous cryotherapy
research is ongoing to determine longer-term clinical outcomes [5,29].
As with any procedure, complications can occur. Complications can be divided into (1) acute, (2) delayed, (3) prolonged-temporary, and (4) permanent. Acute complications include headache, pain, and blister formation. Delayed complications include hemorrhage, infection, and excessive granulation tissue formation. Prolonged-temporary complications include milia, hyperpigmentation, and change in sensation. Permanent complications include alopecia, atrophy, keloids, scarring, hypopigmentation, and ectropion (outward turning of part or the entire eyelid margin away from the eye) formation [17,34].
A minimally invasive treatment that uses extreme cold in the form of liquid nitrogen or argon gas to freeze and destroy diseased tissue, including cancer cells. However, cryosurgery does have risks, but they’re considered lower than other cancer treatments, such as surgery and radiation [30,40]. The risks associated with cryosurgery include blisters, damage to nearby healthy tissue or vessels, infection, a loss of sensation if nerves are affected, pain, scarring, sexual dysfunction, ulcers, and white skin at the site of the surgery [1,2].
Normal biologic reactions to freezing include swelling, bleeding, necrosis, depigmentation, and odor of varying degrees. Swelling occurs within hours of freezing because of increased vascular permeability and vasodilation. This is usually self-limiting and resolves in 48 hours. When lesions are biopsied or ulcerated and undergo cryotherapy, vasodilation after freezing can cause hemorrhage to become more obvious and may become cosmetically objectionable to an owner. Therefore, some form of hemostasis should be used during the biopsy procedure and on ulcerated lesions [11].
Necrosis occurs in 14 to 21 days. The wound contracts and epithelializes under a dry eschar that forms over the necrotic tissue. When the eschar sloughs, it usually reveals healthy granulation tissue or recurrence of the tumor. Because melanocytes and hair follicles are destroyed by freezing, the skin will show depigmentation and will not regrow hair. Owners need to be advised of this before treatment. Offensive odors accompany necrosis of large tumors: cleansing of the area daily and excision of the necrotic tissue may be necessary to ameliorate this problem. Freezing cortical bone causes cell destruction and reduces the strength of the bone by 70% [41].
Spontaneous fractures have been reported months after cryotherapy treatment. Additionally, bone tumors do not respond well to cryotherapy due to their location and composition of bone sturuture, although aneurysmal bone cyst recurrence has been suppressed in people with cryotherapy used as an adjuvant to curettage [42]. Auricular cartilage does not respond well to cryotherapy either and can result in shortening or deformity of an ear. Therefore, cryotherapy should be used on skin tumors in the ears with caution [3].
Hypopigmentation is a common complication with cryotherapy. This complication is due to the temperaturesensitive melanocytes, which die at the relatively high temperature of -5°C to -10°C. Feathering, the process of gradually and lightly freezing the area surrounding the ice ball to prevent an abrupt edge, can provide better cosmetic results [13].
Skin discomfort, generally a burning sensation, occurs with cryosurgery, but the intensity is variable. The most sensitive areas are the fingertips, ears, and temples. Freezing of lesions on the forehead or temple may produce headaches. Treatment in hair-bearing areas can result in permanent hair loss. Hypopigmentation is common, especially with longer freeze times, but is less noticeable in light-skinned patients and improves within several months [6].
Like any percutaneous procedure, bleeding may result-both in the puncture and the freezing of tissues such
as the liver, kidney, or lung. Besides, the damage to normal structures may occur. During liver cryotherapy,
the bile ducts may be injured. During kidney cryotherapy, the ureter or collecting system may be damaged.
The rectum may be damaged during prostate cryotherapy. Any treatment of the abdomen may result in
damage to the bowel and cause a hole in the bowel, which may release bowel contents into the abdomen that
can lead to potentially life-threatening infections [2].
The origins of cryotherapy in human medicine began in the 1960s, but enthusiasm for its use in cancer
treatment dissipated in the 1980s. While cryosurgery is not a new modality, the tools used to deliver the
required temperature change are evolving, making the target areas very precise. This enhanced precision
reduces collateral tissue damage, leading to faster healing and less scarring. The capacity to achieve this
precision is also what makes the biggest difference in treating smaller lesions. The specific unit I is the
CryoProbe X+, which runs at about -127°F. This specific model includes five separate tip sizes that can be
used to match the lesion being treated and can be operated with available 8g and 16g cartridges [11,39].
The precision of the micro-applicator tips prevents the damage to the nearby healthy tissue, resulting in no discomfort to the patient. As such, treatments are very controlled and can be longer in duration if necessary. Moreover, technological advancement in three areas has led to a renaissance in the interest in cryotherapy [3]. These advances are (1) intraoperative ultrasonography, as a technique for monitoring the tissue freezing process, (2) improved cryosurgical equipment, such as vacuum-insulated small-diameter probes supercooled to -200°C, and (3) advances in instrumentation in minimally invasive surgery. Additionally, the discovery that cryotreated tumor tissues are biophysically altered to allow enhancement of chemotherapy transport has sparked interest in combined cancer therapy [43]. Although these techniques are unlikely to be adopted into equine surgical practice anytime soon, because cancer is not a predominant problem in horses, some of these advances will make it into the hands of the equine surgeon as minimally invasive techniques become more commonplace [23].
Conclusion
Cryosurgery is a type of surgery that involves the use of extreme cold to destroy abnormal tissues, such
as tumors. The surgery most often involves the use of liquid nitrogen, although carbon dioxide and argon
may also be used. Cryotherapy is safe and easy to use treatment to destroy many benign and malignant
lesions. The cryotherapy operator must be aware of the complications associated with this modality; for
example, a complication is a hypopigmentation (dark-skinned individuals are prone to hypopigmentation).
Veterinary practices today are filled with innovative technological advancements that assist us in effectively
treating patients ethically and compassionately. While traditional surgical and medical practices will always
provide the foundation for our therapies, additional modalities such as cryosurgery also have their place.
Cryosurgery provides another option for commonly-seen dermatological lesions that is quick, effective, less
invasive, and requires little to no anesthesia. Cryosurgery has been readily accepted by my clientele and well
tolerated by my patients, making it a great fit for my practice. One needs to be aware of the fact that lesions
may need to be treated multiple times before resolution. In conclusion, cryosurgery is less invasive, with no
post-operative care; bloodless surgery, and does not require sutures and cones; thus, a wonderful benefit for
both patients and caregivers.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!