Biography
Interests
Halil Yanardag1, Cuneyt Tetikkurt2*, Cigdem Papila3, Muammer Bilir1 & Seza Tetikkurt4
1Department of Internal Medicine Medicine, Cerrahpasa Medical Faculty, Istanbul Cerrahpasa University, Turkey
2Department of Pulmonary Medicine, Cerrahpasa Medical Faculty, Istanbul Cerrahpasa University, Turkey
3Department of Oncology, Cerrahpasa Medical Faculty, Istanbul Cerrahpasa University, Turkey
4Department of Pathology, Demiroglu Bilim University Medical Faculty, Turkey
*Correspondence to: Dr. Cuneyt Tetikkurt, Department of Pulmonary Medicine, Cerrahpasa Medical Faculty, Istanbul Cerrahpasa University, Turkey.
Copyright © 2019 Dr. Cuneyt Tetikkurt, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Survival of lung cancer patients are affected by many different factors that are extremely variable for each individual. The prognosis of lung cancer primarily depends upon the tumor type, patient immunity and treatment. The objective of our study was to determine the influence of patient symptoms, laboratory findings, imaging modalities and histopathologic tumor features on the prognostic outcome of lung cancer patients. The second aim was to evaluate whether the collaboration of these data predicted an accurate prognostic assessment.
A total of 1833 lung cancer patients evaluated at our clinic between 1984 and 2005 years were included in the study. The patients had blood chemistry, urine analysis, chest x-ray, pulmonary function tests, chest computed tomography and histopathologic verification of the lung cancer including tumor differentiation with microscopic features relevant to airway, nerve, blood or lymphatic vessel invasion. The prognostic outcome of the patients were appraised in regard to initial clinical symptoms, performance status, laboratory findings, pulmonary function test results, radiologic and histopathologic manifestations. BODE and Karnofsky index were used to determine patient performance. Pearson correlation and chi-square tests were used for statistical analysis.
Weight loss, hemoptysis and lassitude were the most common presenting symptoms. Patient performance status and symptom analysis revealed a weak correlation with prognosis. The preliminary laboratory findings and the pulmonary function test results were also incoherent predictors of survival. Imaging findings including chest x-ray and computed tomography manifestations displayed a noteworthy correlation for the prognostic outcome. Analysis of the histopathologic manifestations including tumor type, differentiation profile and microscopic invasive features of the airways, nerves, blood or lymphatic vessels revealed the most significant prognostic correlation among our patients. The aforementioned criteria showed a poor or a moderate correlation by themselves in regard to an accurate prognostic assessment. Collaboration of the clinical manifestations revealed a significantly high correlation and an indisputable evaluation for the prognostic outcome of lung cancer patients. Since the pathogenesis of lung cancer involves many different complex mechanisms, the more and the specific clinical data of the individual patient are collaborated, the more accurate and the definitive prognostic assessment will come out for lung cancer.
Introduction
Lung cancer was a rare disease at the start of the twentieth century but exposure to carcinogenic agents has
caused lung cancer to become a pandemic. It is the most lethal cancer among both men and women in the
United States. Lung cancer is also most frequent cause of cancer death among men, is the second foremost of
death among all cancer types and is the second leading cause of cancer death among women worldwide that
causes 1.6 million deaths annually which is more than the total number of mortality due to breast, prostate
and colon cancer [1-5]. Primary lung cancer has a heterogeneous structure in terms of clinical presentation,
histopathology, course, response to treatment and prognosis that may show significant differences among
the individual patients. The survival for lung cancer is most dependent on the stage and the histopathologic
features of the tumor. Clinicians should keep in mind that not everyone with a particular stage of lung
cancer exhibits an identical treatment response or the same prognosis. No matter how advanced the lung
cancer staging is, there are serious inadequacies in determining the prognostic outcome and directing the
treatment modalities. In addition, clinicians may be confronted with a number of additional conditions, such
as infections, treatment complications and comorbid diseases that may play a significant role on the patient
survival.
As lung cancer usually becomes symptomatic and clinically evident late in the course of the disease, the curative treatment is not possible in most of the patients. The overall 5-year survival with lung cancer is only 11.2% for men and is 13.9% for women [6-8]. Current studies target to identify patients with early-stage disease to improve life expectancy. Prolonging survival and improving quality of life for patients presenting with advanced lung cancer are also the main concerns of today’s research. In these circumstances, studies on the prediction of survival gain great importance that provide guidance in terms of both prognosis and treatment options for the patients. The aim of our study is to evaluate the significance of initial symptoms, performance status, pathologic tumor features, laboratory and radiologic manifestations on the prognostic outcome of lung cancer patients. The second objective is to establish whether the collaboration of these findings provide a more accurate prognostic assessment than the conventional staging system.
Materials and Methods
A total of 1833 lung cancer patients admitted at the Cerrahpasa Medical Faculty between 1984 August
and 2005 November years were included in this study. Of these patients 1657 (90.4%) were males and 176
(9.6%) were females. The mean age was 58.7±19.4 years. Dermographic features of the patients including
age, gender, the laboratory findings and the pulmonary function test results are depicted in Table 1. Tumors
metastatic to the lung, primary pleural, thoracic wall tumors and patients with comorbid diseaes were
excluded from the study. Two hundred thirty six patients (236/1833; 12.8%) were non-smokers while 143
(142/1833; 7.7%) were passive smokers. Performance status of the patients was determined by using the
BODE dyspnea and Karnofsky index. The initial clinical manifestations including age, gender, symptoms,
laboratory findings, radiologic manifestations and histopathologic findings were assessed in regard to the
prognostic outcome of the patients.
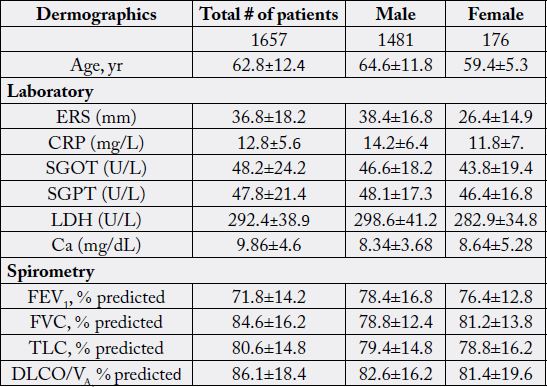
Data are presented as mean±SD or %.
Patients with metastatic disease were identified by symptoms, laboratory, bone scintigraphy, CT and MR. Specific organ metastasis including brain, liver, bone and adrenal glands was evaluated by neurology and internal medicine consultation. The mean follow-up was 124.2±16.8 months. Clinical manifestations of the lung cancer patients is shown in Table 2. For statistical analysis, Pearson correlation test and chi-square were used. Pearson test was used to determine the correlation between different variables. A correlation coefficient was designated as weak (r <0.3), intermediate (0.3≤ r ≤ 0.7) and strong (r >0.7). A p value less than 0.5 was accepted as statistically significant. The prognostic evaluation was asessed according to the survival time following initial diagnosis. The prognostic outcome was designated as poor (12-36 months), intermediate (36-60 months) and good prognosis (more than 60 months) in regard to survival time after the initial diagnosis of lung cancer. The statistical analysis and the correlation status of the patients including gender, age, initial symptoms, patient performance index, pulmonary function tests, chest radiology, thorax computed tomography and histopathological findings in regard to prognosis is depicted in Table 3.
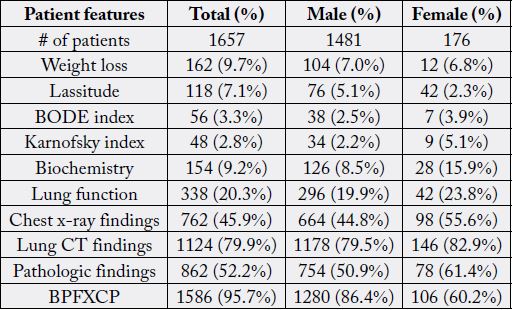
B: Biochemistry: P: performance, F: pulmonary function, X: chest x-ray, C: CT and P: pathology.
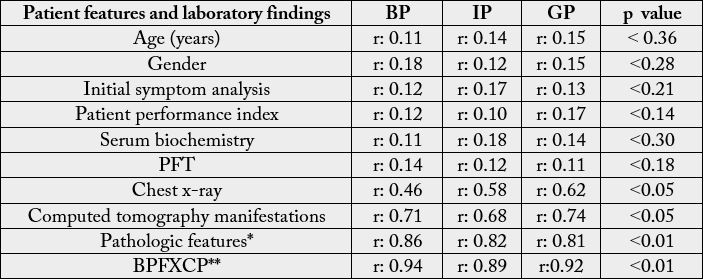
BPFXCP: collaboration of clinical and laboratory findings. χ2 test, ANOVA, Kruskal-Wallis test and Bonferronni corrected two way Mann-Whitney test were used for statistical analysis. Pearson test was used for correlation analysis between prognosis and different clinical variables. PP: poor prognosis, IP: intermediate prognosis, GP: good prognosis
Results
The most frequent lung cancer was adenocarcinoma (39.4%, p<0.05 ) followed by squamous cell carcinoma
(36.2%) among smokers. Adenocarcinoma had the highest incidence (24.6%, p<0.05) followed by squamous
carcinoma (21.8%, p<0.05) among non-smokers. A significant correlation was found in regard to tumor
location between smokers and non-smokers. In non-smokers, the tumor was located in the left peripheral
and left proximal areas in 62.8% of the patients while in smokers the right peripheral and righ proximal
locations were more frequent (58.6%). Adenocarcinoma was most frequent in both peripheral lung zones
while bronchioloalveolar carcinoma was more comon in the right peripheral zone. Adenocarcinoma incidence
was significantly (p< 0.05) higher in the female patients. Gender and age had no significant effect on the lung
cancer prognosis (Table 3). Weight loss (more than 6kg in the last three months), hemoptysis and lassitude
were the most frequent initial symptoms but their prognostic correlation was poor and insignificant. BODE
and Karnofsky index were indeterminate indicators for survival. Of the laboratory findings, the initial high
serum calcium levels and increased serum lactate dehydrogenase levels were the most frequent and crucial
laboratory findings while their correlation with prognosis was weak (Table 3).
Chest x-ray and computed tomography of the thorax findings revealing a tumor size (> 3.0 cm), irregular or spicular tumor borders and a cavity wall thickness more than 15mm were noteworthy manifestations for prognosis with a high correlation (Table 3). Lower lobe tumors showed the worst prognosis (r: 0.78, p< 0.05) in regard to tumor site. Patients with small cell carcinoma followed by large cell and adenocarcinoma revealed the worst prognostic outcome (r: 0.95; p< 0.01, r: 0.89; p< 0.01 and r: 0.82; p< 0.01) in regard to histopathologic tumor type. The prognosis showed an increasing poor trend along with the higher stages and the histopathologic tumor features. Histopathological examination of tumor differentiation, microscopic airway, nerve, blood and lymphatic vessel invasion provided the most accurate correlation for prognosis. The best prognostic assessment of the lung cancer patients was obtained when all the aforemetioned criteria including the initial symptoms, the laboratory, the radiologic and the histopathologic findings were collaborated. This new assessment protocole provided an accurate and a definitive appraisement for the prognostic evaluation of the lung cancer patients.
Discussion
There are many factors effecting the prognostic outcome and the survival of the lung cancer patients.
Genetics, immunity, comorbid diseases, tumor stage, tumor type and differentiation status appear to be the
most significant determinants of lung cancer prognosis. Because of the fact that many factors play an active
role in prognosis, survival determination will reveal less explicit results if prognostic evaluation is done by depending only on the conventional staging system. Our findings indicate that the most definite prognostic
evaluation can only be achieved by the collaboration of all the relevant clinical factors including the
laboratory results, patient symptoms, radiologic findings and pathologic manifestations including tumor
type, differentiation and histopathologic features. These criteria were not statistically significant for prognosis
by themselves and revealed a weak or a moderate correlation on their own while liaison of all these factors in
unison exhibited an undisputable assessment for the prognostic outcome of lung cancer patients.
Radiologically, higher tumor size and presence of spicular extensions on chest x-ray and CT exhibited a significant negative prognostic factor. Pathologically vascular, lymphatic or nerve invasion was an extremely crucial and definitive histopathologic features for poor prognosis. The undifferentiated tumors and small cell carcinoma followed by adenocarcinoma showed the worse prognostic outcome. The most explicit indicator of tumor prognosis was obtained by evaluating all of the above criteria together. Since prognosis of lung cancer is associated with many factors, the accurate assessment of prognosis will be more successful if multiple criteria are considered to determine the outcome of lung cancer patients as the results of our study indicated.
The more pronounced the undifferentiated status of the tumor, the greater will be the tumor aggressiveness leading to poor survival. The tumor type is another well predictor of survival that was the worst prognostic factor for the small cell carcinoma type. CT findings of higher dimension including axial and sagittal diameters with the spicular extensions are the hallmark of advanced tumor that were associated with a poor patient outcome. Pathological evidence of vascular, lymphatic or nerve invasion by tumor cells is another indicator for decreased survival revealing an advanced disease status that indicates the microscopic tumor cell metastasis. The collaboration of tumor associated factors we put forward determined the prognostic outcome of lung cancer patients more accurately. As these factors are in a way indirect indicators of advanced or aggressive disease that reveal the different aspects of the tumor metastasis which is the hallmark of survival, their collaboration established the prognostic outcome accurately. The laboratory results revealed a weak and unsignificant correlation with the prognosis of the lung cancer patients due to their low sensitivity and specificity that may be associated with many other diseases. Because the tumor pathogenesis and development is very complex it is obvious that as more factors are included for evaluation the more accurate the prognostic assessment will be.
The small patient population may be considered as the weak side of our study. We are aware of the fact that multiple factors play a prognostic role in the survival of lung cancer patients. Our study consisted of only Caucassian people. Therefore, it is a necessity to perform multiple studies with large population sizes including as many as different patient types with discriminating genetic features. The pathogenesis of lung cancer is very complex and depends upon many factors including genetic, tumor type, patient immunity along with social and environmental features. Due to this complex tumor structure we believe that prognostic studies would establish more accurate results if and only if more relevant criteria are evaluated such as the molecular or genetic pathways of lung cancer since they are involved in cancer cell proliferation. Currently only a few of the numerous identified molecular targets are used in clinical practice outside the clinical trials. Molecular biology of lung carcinogenesis is the hallmark of patient prognosis. For lung cancer treatment, blocking tumor growth by targeting the surrounding angiogenesis, pro-tumorigenic growth factor activation, anti-apoptotic cascades and other cancer-promoting signal transduction events are extremely important. The heterogeneous complex nature of lung cancer makes it difficult for us to interpret and predict the prognostic outcome accurately. Data obtained in very diverse patient populations would provide new insights to tumor prognosis. As the lung cancer histopathology and molecular pathways are delineated, we would be able to stratify patients based on a refined understanding of their histology that may more uniquely ascertain the patient prognosis. PET findings, standart uptake value (SUV) and molecular genetic findings were not included as these modalities were unavailable at the time of our study. Another missing aspect of our study may be considered as the exclusion of individuals with comorbid diseases. Since comorbid diseases effect patient survival independant of lung cancer, their exclusion would only reveal the absolute effect of lung cancer on survival thereby providing a more accurate determination of prognosis.
The targeted treatment in lung cancer aims for specific genes, proteins or the tissue environment that contributes to cancer growth and survival. The treatment includes inhibiting angiogenesis, epidermal growth factor receptor and other genes like ALK, ROS1 and NRTK while immunotherapy restores the immune function of the patient. As these treatment modalities indicate lung cancer is a complex disease consisting of multiple pathogenetic mechanisms. It is well known that some of the patients respond to these treatment options while in others there is no response. A similar treatment response is encountered in the treatment of lung cancer with conventional drug treatment. This clinical profile clearly demonstrates how complex the pathogenesis of lung cancer is. Our study results suggest that to determine an accurate prognostic assessment in lung cancer patients all available clinical factors should be considered and appraised. It is well kown that as the number of tests used for diagnosis increases, the diagnostic specificity and sensitivity will reach to more unequivocal consequences.
It is well known that there are some significant limitations of the new TNM staging system [9-12]. The data lacks details in regard to details of the tumor structure and radiologic manifestations including lymphangitis carcinomatosis. The standart uptake value (SUV) has not been incorporated as well as the absence of data for immunohistochemistry and molecular genetics [13,14]. Tumor size and the other vital CT manifestations of lung cancer are not included in the current TNM system that appears to be a fundamental deficiency. The new TNM system lacks specific evaluation of the histopathologic tumor features like lymphatic and blood vessel infiltration of tumor cells which appears to be one of the most crucial and noteworthy determinant for the survival of lung cancer patients. Consequently, the current TNM system has limited utility for estimating the prognostic outcome of lung cancer [15,16]. The main reason for this insufficient determinant of prognostic assessment is the lack of specific patient data in individual bases that should have included every detail of the patient profile. Our prognostic assessment protocol includes the most crucial factors for an unequivocal assessment of the prognostic outcome of the lung cancer patients. Another important aspect of this study is, the more individualization of personal profile and the more specific data entry for any patient, the more accurate and the more definitive the prognostic assessment will be.
Conclusions
Lung cancer is a complex disease including many different pathogenetic mechanisms for its development,
clinical course, treatment response and patient survival. Consequently, the prognostic outcome of the
individual patient depends upon numerous factors involving tumor type and differentiation, treatment options, immune and genetic status of the patient. The presence of such different and variable factors relevant
to tumor pathogenesis makes the prognostic evaluation of the patient extremely difficult. This study has
clearly demonstrated that for an accurate survival and prognostic assessment, an individual patient analysis
should be performed including the greatest number of available factors comprising all the clinical, laboratory,
pathology and imaging findings available. Analysis of the histopathologic tumor features revealed the most
noteworthy correlation with prognosis followed by the chest computed tomography findings among our
patients. The accuracy of prognostic survival assessment will increase depending on the number of the
clinical criteria of the individual patient that has been incorporated. The more unique and the more specific
clinical factors are individually appraised, the more accurate and definitive the prognostic assessment of lung
cancer prognosis will be. The prognostic outcome assessment of lung cancer should be tailored according
to the individual patient profile. This new assessment protocole has produced significantly accurate and
undeniable data for an unequivocal evaluation for the prognostic outcome of the lung cancer patients.
Author Contributions
Halil Yanardag has performed patient data collection and evaluation.
Cuneyt Tetikkurt has designed the study and wrote the manuscript.
Cigdem Papila has contributed to organization of patient files and preparation of references.
Muammer Bilir has performed the statistical analysis.
Seza Tetikkurt reviewed the pathologic aspects of lung cancer.
Conflicts of Interest
Halil Yanardag does not have any conflicts of interest to declare associated with this study.
Cuneyt Tetikkurt does not have any conflicts of interest to declare associated with this study.
Cigdem Papila does not have any conflicts of interest to declare associated with this study.
Muammer Bilir does not have any conflicts of interest to declare associated with this study.
Seza Tetikkurt does not have any conflicts of interest to declare associated with this study.
Further Information
There is no funding and poster presentation associated with this study.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!